Abstract
Objectives
To retrospectively investigate whether the baseline extent and 1-year change in regional disease patterns on CT can predict survival of patients with idiopathic pulmonary fibrosis (IPF).
Methods
A total of 144 IPF patients with CT scans at the time of diagnosis and 1 year later were included. The extents of five regional disease patterns were quantified using an in-house texture-based automated system. The fibrosis score was defined as the sum of the extent of honeycombing and reticular opacity. The Cox proportional hazard model was used to determine the independent predictors of survival.
Results
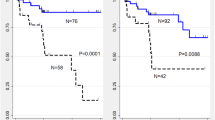
A total of 106 patients (73.6%) died during the follow-up period. Univariate analysis revealed that age, baseline forced vital capacity, total lung capacity, diffusing capacity of the lung for carbon monoxide, six-minute walk distance, desaturation, honeycombing, reticular opacity, fibrosis score, and interval changes in honeycombing and fibrosis score were significantly associated with survival. Multivariate analysis revealed that age, desaturation, fibrosis score and interval change in fibrosis score were significant independent predictors of survival (p = 0.003, <0.001, 0.001 and <0.001). The C-index for the developed model was 0.768.
Conclusion
Texture-based, automated CT quantification of fibrosis can be used as an independent predictor of survival in IPF patients.
Key Points
• Automated quantified fibrosis on CT was a significant predictor of survival.
• Automated quantified interval change in fibrosis on CT was an independent predictor.
• The predictive model showed comparable discriminative power with a C-index of 0.768.
• Automated CT quantification can be considered to evaluate prognosis in routine practice.


Similar content being viewed by others
Abbreviations
- 6MWD:
-
six-minute walk distance
- CONS:
-
consolidation
- DLCO :
-
diffusing capacity of the lung for carbon monoxide
- EMPH:
-
emphysema
- FVC:
-
forced vital capacity
- GAP:
-
gender, age, and physiology
- GGO:
-
ground-glass opacity
- HC:
-
honeycombing
- IPF:
-
idiopathic pulmonary fibrosis
- NL:
-
normal
- PFT:
-
pulmonary function test
- RO:
-
reticular opacity
- ROI:
-
region of interest
- SpO2 :
-
oxyhaemoglobin saturation
- TLC:
-
total lung capacity
References
Raghu G, Collard HR, Egan JJ et al (2011) An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183:788–824
Martinez FJ, Safrin S, Weycker D et al (2005) The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med 142:963–967
Fernandez Perez ER, Daniels CE, Schroeder DR et al (2010) Incidence, prevalence, and clinical course of idiopathic pulmonary fibrosis: a population-based study. Chest 137:129–137
Ley B, Collard HR, King TE Jr (2011) Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 183:431–440
du Bois RM, Weycker D, Albera C et al (2011) Forced vital capacity in patients with idiopathic pulmonary fibrosis: test properties and minimal clinically important difference. Am J Respir Crit Care Med 184:1382–1389
Flaherty KR, Mumford JA, Murray S et al (2003) Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Respir Crit Care Med 168:543–548
Zappala CJ, Latsi PI, Nicholson AG et al (2010) Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. Eur Respir J 35:830–836
Noble PW, Albera C, Bradford WZ et al (2011) Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 377:1760–1769
King TE Jr, Bradford WZ, Castro-Bernardini S et al (2014) A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 370:2083–2092
Richeldi L, du Bois RM, Raghu G et al (2014) Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 370:2071–2082
Pellegrino R, Viegi G, Brusasco V et al (2005) Interpretative strategies for lung function tests. Eur Respir J 26:948–968
Cottin V, Cordier JF (2009) The syndrome of combined pulmonary fibrosis and emphysema. Chest 136:1–2
Lynch DA, Godwin JD, Safrin S et al (2005) High-resolution computed tomography in idiopathic pulmonary fibrosis: diagnosis and prognosis. Am J Respir Crit Care Med 172:488–493
Sumikawa H, Johkoh T, Colby TV et al (2008) Computed tomography findings in pathological usual interstitial pneumonia: relationship to survival. Am J Respir Crit Care Med 177:433–439
Best AC, Meng J, Lynch AM et al (2008) Idiopathic pulmonary fibrosis: physiologic tests, quantitative CT indexes, and CT visual scores as predictors of mortality. Radiology 246:935–940
Ley B, Elicker BM, Hartman TE et al (2014) Idiopathic pulmonary fibrosis: CT and risk of death. Radiology 273:570–579
Watadani T, Sakai F, Johkoh T et al (2013) Interobserver variability in the CT assessment of honeycombing in the lungs. Radiology 266:936–944
Walsh SL, Calandriello L, Sverzellati N, Wells AU, Hansell DM, Consort UIPO (2016) Interobserver agreement for the ATS/ERS/JRS/ALAT criteria for a UIP pattern on CT. Thorax 71:45–51
Maldonado F, Moua T, Rajagopalan S et al (2014) Automated quantification of radiological patterns predicts survival in idiopathic pulmonary fibrosis. Eur Respir J 43:204–212
Yoon RG, Seo JB, Kim N et al (2013) Quantitative assessment of change in regional disease patterns on serial HRCT of fibrotic interstitial pneumonia with texture-based automated quantification system. Eur Radiol 23:692–701
Park HJ, Lee SM, Song JW et al (2016) Texture-based automated quantitative assessment of regional patterns on initial CT in patients with idiopathic pulmonary fibrosis: relationship to decline in forced vital capacity. AJR Am J Roentgenol 207:976–983
Miller MR, Hankinson J, Brusasco V et al (2005) Standardisation of spirometry. Eur Respir J 26:319–338
Lama VN, Flaherty KR, Toews GB et al (2003) Prognostic value of desaturation during a 6-minutes walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med 168:1084–1090
Park SO, Seo JB, Kim N et al (2009) Feasibility of automated quantification of regional disease patterns depicted on high-resolution computed tomography in patients with various diffuse lung diseases. Korean J Radiol 10:455–463
Park SO, Seo JB, Kim N, Lee YK, Lee J, Kim DS (2011) Comparison of usual interstitial pneumonia and nonspecific interstitial pneumonia: quantification of disease severity and discrimination between two diseases on HRCT using a texture-based automated system. Korean J Radiol 12:297–307
Chang Y, Lim J, Kim N, Seo JB, Lynch DA (2013) A support vector machine classifier reduces interscanner variation in the HRCT classification of regional disease pattern in diffuse lung disease: comparison to a Bayesian classifier. Med Phys 40:051912
Harrell FE Jr, Lee KL, Mark DB (1996) Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15:361–387
Wells AU, Desai SR, Rubens MB et al (2003) Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med 167:962–969
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Joon Beom Seo.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Funding
This study was supported by a grant (2016-7014) from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the institutional review board.
Ethical approval
Institutional review board approval was obtained.
Study subjects or cohorts overlap
The 144 patients of our study were identical to the study population in the previous report [21]. While the previous study assessed predictive factors for decline in FVC on initial CT using texture-based automated quantification in IPF, this work focused on whether baseline extent and 1-year change in regional disease patterns on CT can be used to predict survival of patients with IPF. Thus, this work is substantially different from the previous report.
Methodology
• retrospective
• observational
• performed at one institution
Rights and permissions
About this article
Cite this article
Lee, S.M., Seo, J.B., Oh, S.Y. et al. Prediction of survival by texture-based automated quantitative assessment of regional disease patterns on CT in idiopathic pulmonary fibrosis. Eur Radiol 28, 1293–1300 (2018). https://doi.org/10.1007/s00330-017-5028-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-5028-0