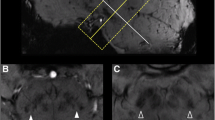
Abstract
Objective
To investigate the value of ‘swallow-tail’ sign and putaminal hypointensity on 3 T susceptibility-weighted imaging (SWI) for distinguishing multiple system atrophy (MSA) from idiopathic Parkinson’s disease (IPD).
Methods
Three groups – 39 MSA patients, 18 IPD patients,and 31 healthy controls (HCs) – were administered a 3 T SWI sequence to evaluate ‘swallow-tail’ sign and putaminal hypointensity using visual scales from 0 to 2 and 0 to 3 scores, respectively. The diagnostic accuracy of the two signs separately and combined was calculated using a receiver operating characteristic curve, with clinical diagnosis as the gold standard.
Results
The scores of ‘swallow-tail’ sign were lower in IPD than in MSA or in HCs, as well as for putaminal hypointensity in IPD or HCs than in MSA (p < 0.05). The sensitivity and specificity of ‘swallow-tail’ sign and putaminal hypointensity were 87.9% and 83.3%, and 35.9% and 100%, respectively, in the respective patient groups. The area under the curve of combined signs was increased from 0.85 (‘swallow tail’) or 0.68 (putaminal hypointensity) to 0.93.
Conclusion
The combination of ‘swallow-tail’ sign and putaminal hypointensity can increase the accuracy of discriminating between MSA and IPD.
Key Points
• Differential diagnosis of MSA and IPD is still challenging in clinical practice.
• Absence of ‘swallow-tail’ sign is a valuable biomarker for IPD on SWI.
• Putaminal hypointensity is a valuable biomarker for MSA on SWI.
• Combined ‘swallow- tail’ sign and putaminal hypointensity increase diagnostic accuracy.



Similar content being viewed by others
Abbreviations
- AP:
-
Atypical Parkinsonism
- AUC:
-
Area under the curve
- HCs:
-
Healthy controls
- IPD:
-
Idiopathic Parkinson’s disease
- MRI:
-
Magnetic resonance imaging
- MSA:
-
Multiple system atrophy
- ROC:
-
Receiver operating characteristic
- SN:
-
Substantia nigra
- SWI:
-
Susceptibility-weighted imaging
References
Stefanova N, Bücke P, Duerr S, Wenning GK (2009) Multiple system atrophy: an update. Lancet Neurol 8:1172–1178
Ramli N, Nair SR, Ramli NM, Lim SY (2015) Differentiating multiple-system atrophy from Parkinson's disease. Clin Radiol 70:555–564
Dabrowska M, Schinwelski M, Sitek EJ et al (2015) The role of neuroimaging in the diagnosis of the atypical parkinsonian syndromes in clinical practice. Neurol Neurochir Pol 49:421–431
Mahlknecht P, Hotter A, Hussl A, Esterhammer R, Schocke M, Seppi K (2010) Significance of MRI in diagnosis and differential diagnosis of Parkinson's disease. Neurodegener Dis 7:300–318
Paviour DC, Thornton JS, Lees AJ, Jager HR (2007) Diffusion-weighted magnetic resonance imaging differentiates Parkinsonian variant of multiple-system atrophy from progressive supranuclear palsy. Mov Disord 22:68–74
Meijer FJ, van Rumund A, Tuladhar AM et al (2015) Conventional 3T brain MRI and diffusion tensor imaging in the diagnostic workup of early stage parkinsonism. Neuroradiology 57:655–669
Gottlich M, Munte TF, Heldmann M, Kasten M, Hagenah J, Kramer UM (2013) Altered resting state brain networks in Parkinson’s disease. PLoS One 8, e77336
You H, Wang J, Wang H et al (2011) Altered regional homogeneity in motor cortices in patients with multiple system atrophy. Neurosci Lett 502:18–23
Francesco F, Isabella LS, Vincenzo L et al (1997) Proton Magnetic Resonance Spectroscopy in Parlunson’s Disease and Atypical Parkinsonian Disorders. Mov Disord 12:903–909
Eckert T, Sailer M, Kaufmann J et al (2004) Differentiation of idiopathic Parkinson's disease, multiple system atrophy, progressive supranuclear palsy, and healthy controls using magnetization transfer imaging. Neuroimage 21:229–235
Sehgal V, Delproposto Z, Haacke EM et al (2005) Clinical applications of neuroimaging with susceptibility-weighted imaging. J Magn Reson Imaging 22:439–450
Liu C, Li W, Tong KA, Yeom KW, Kuzminski S (2015) Susceptibility-weighted imaging and quantitative susceptibility mapping in the brain. J Magn Reson Imaging 42:23–41
Wang C, Fan G, Xu K, Wang S (2013) Quantitative assessment of iron deposition in the midbrain using 3D-enhanced T2 star weighted angiography (ESWAN): a preliminary cross-sectional study of 20 Parkinson's disease patients. Magn Reson Imaging 31:1068–1073
Gupta D, Saini J, Kesavadas C, Sarma PS, Kishore A (2010) Utility of susceptibility-weighted MRI in differentiating Parkinson's disease and atypical parkinsonism. Neuroradiology 52:1087–1094
Schwarz ST, Afzal M, Morgan PS, Bajaj N, Gowland PA, Auer DP (2014) The 'swallow tail' appearance of the healthy nigrosome - a new accurate test of Parkinson's disease: a case-control and retrospective cross-sectional MRI study at 3T. PLoS One 9, e93814
Meijer FJ, Steens SC, van Rumund A et al (2016) Nigrosome-1 on Susceptibility Weighted Imaging to Differentiate Parkinson's Disease From Atypical Parkinsonism: An In Vivo and Ex Vivo Pilot Study. Pol J Radiol 81:363–369
Wang Y, Butros SR, Shuai X et al (2012) Different iron-deposition patterns of multiple system atrophy with predominant parkinsonism and idiopathetic Parkinson diseases demonstrated by phase-corrected susceptibility-weighted imaging. AJNR Am J Neuroradiol 33:266–273
Jae-hyeok L, Seung-kug B (2011) Putaminal hypointensity in the parkinsonianvariant of multiple system atrophy: simple visual assessment using susceptibility-weighted imaging. J Mov Disord 4:60–63
Meijer FJ, van Rumund A, Fasen BA et al (2015) Susceptibility-weighted imaging improves the diagnostic accuracy of 3T brain MRI in the work-up of parkinsonism. AJNR Am J Neuroradiol 36:454–460
Gilman S, Wenning GK, Low PA et al (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71:670–676
Andrew JH, Susan ED, Linda K et al (1992) Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Gao P, Zhou PY, Wang PQ et al (2016) Universality analysis of the existence of substantia nigra ‘swallow tail’ appearance of non-Parkinson patients in 3T SWI. Eur Rev Med Pharmacol Sci 20:1307–1314
Damier P, Hirsch EC, Agid Y et al (1999) The substantia nigra of the humanbrain I. Nigrosomes and the nigral matrix, a compartmental organization based on calbindin D28K immunohistochemistry. Brain 122:1421–1436
Anna IB, Stefan TS, Alain P et al (2013) Visualization of nigrosome 1 and its loss in PD: Pathoanatomical correlation and in vivo 7 T MRI. Neurology 81:534–540
Fearnley JM, Lees RJ (1991) Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain 114:2283–2301
Sian-Hulsmann J, Mandel S, Youdim MB, Riederer P (2011) The relevance of iron in the pathogenesis of Parkinson's disease. J Neurochem 118:939–957
Zecca L, Casella L, Albertini A et al (2008) Neuromelanin can protect against iron-mediated oxidative damage in system modeling iron overload of brain aging and Parkinson's disease. J Neurochem 106:1866–1875
Yoon RG, Kim SJ, Kim HS et al (2015) The utility of susceptibility-weighted imaging for differentiating Parkinsonism-predominant multiple system atrophy from Parkinson's disease: correlation with 18F-flurodeoxyglucose positron-emission tomography. Neurosci Lett 584:296–301
Han YH, Lee JH, Kang BM et al (2013) Topographical differences of brain iron deposition between progressive supranuclear palsy and parkinsonian variant multiple system atrophy. J Neurol Sci 325:29–35
Matsusue E, Fujii S, Kanasaki Y et al (2008) Putaminal lesion in multiple system atrophy: postmortem MR-pathological correlations. Neuroradiology 50:559–567
Sugiyama A, Ito S, Suichi T et al (2015) Putaminal hypointensity on T2*-weighted MR imaging is the most practically useful sign in diagnosing multiple system atrophy: A preliminary study. J Neurol Sci 349:174–178
Harder SL, Hopp KM, Ward H, Neglio H, Gitlin J, Kido D (2008) Mineralization of the deep gray matter with age: a retrospective review with susceptibility-weighted MR imaging. AJNR Am J Neuroradiol 29:176–183
Zhang J, Wei L, Hu X et al (2015) Akinetic-rigid and tremor-dominant Parkinson's disease patients show different patterns of intrinsic brain activity. Parkinsonism Relat Disord 21:23–30
Wenning GK, Tison F, Shlomo YB et al (1997) Multiple System Atrophy: A Review of 203 Pathologically Proven Cases. Mov Disord 12:133–147
Acknowledgements
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Guoguang Fan.
Conflict of interest and funding
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
No study subjects or cohorts have been previously reported.
Methodology
-
retrospective
-
diagnostic or prognostic study
-
performed at one institution
Rights and permissions
About this article
Cite this article
Wang, N., Yang, H., Li, C. et al. Using ‘swallow-tail’ sign and putaminal hypointensity as biomarkers to distinguish multiple system atrophy from idiopathic Parkinson’s disease: A susceptibility-weighted imaging study. Eur Radiol 27, 3174–3180 (2017). https://doi.org/10.1007/s00330-017-4743-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-4743-x