Abstract
Objectives
Our aim was to investigate regional difference in brain activities in response to antiepileptic drug (AED) medications in benign epilepsy with central-temporal spikes (BECTS) using resting-state functional magnetic resonance imaging (fMRI).
Methods
Fifty-seven patients with BECTS underwent resting-state fMRI scans after receiving either valproic acid (VPA) (n = 15), levetiracetam (LEV) (n = 21), or no medication (n = 21). fMRI regional homogeneity (ReHo) parameter among the three groups of patients were compared and were correlated with total doses of AED in the two medicated groups.
Results
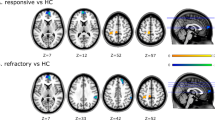
Compared with patients on no-medication, patients receiving either VPA or LEV showed decreased ReHo in the central-temporal region, frontal cortex, and thalamus. In particular, the VPA group showed greater ReHo decrease in the thalamus and milder in cortices and caudate heads compared with the LEV group. In addition, the VPA group demonstrated a negative correlation between ReHo values in the central-temporal region and medication dose.
Conclusion
Both VPA and LEV inhibit resting-state neural activity in the central-temporal region, which is the main epileptogenic focus of BECTS. VPA reduced brain activity in the cortical epileptogenic regions and thalamus evenly, whereas LEV reduced brain activity predominantly in the cortices. Interestingly, VPA showed a cumulative effect on inhibiting brain activity in the epileptogenic regions in BECTS.
Key Points
• Regional differences in brain activity in response to different AEDs in BECTS.
• AEDs inhibit resting-state neural activity in epileptogenic and subcortical regions in BECTS.
• Valproic acid effect on the cortical epileptogenic regions and thalamus evenly.
• Levetiracetam effect seen predominantly in cortices.
• Valproic acid has a cumulative effect on inhibiting brain activity in epileptogenic regions.



Similar content being viewed by others
Abbreviations
- BECTS:
-
benign epilepsy with central-temporal spikes
- AED:
-
Antiepileptic drug
- VPA:
-
Valproic acid
- LEV:
-
Levetiracetam
- ReHo:
-
Regional homogeneity
- EEG-fMRI:
-
Simultaneous electroencephalogram and functional magnetic resonance imaging
References
Beaussart M (1972) Benign epilepsy of children with Rolandic (centro-temporal) paroxysmal foci. A clinical entity. Study of 221 cases. Epilepsia 13(6):795–811
Giordani B, Caveney AF, Laughrin D, Huffman JL, Berent S, Sharma U, Giles JM, Garofalo EA (2006) Cognition and behavior in children with benign epilepsy with centrotemporal spikes (BECTS). Epilepsy Res 70(1):89–94
Stephani U (2000) Typical semiology of benign childhood epilepsy with centrotemporal spikes (BCECTS). Epileptic Disord: Int Epilepsy J Videotape 2(Suppl 1):S3–S4
Glauser TA, Ayala R, Elterman RD, Mitchell WG, Van Orman CB, Gauer LJ, Lu Z, Group NS (2006) Double-blind placebo-controlled trial of adjunctive levetiracetam in pediatric partial seizures. Neurology 66(11):1654–1660
Loscher W (2002) Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS drugs 16(10):669–694
Kaminski RM, Matagne A, Leclercq K, Gillard M, Michel P, Kenda B, Talaga P, Klitgaard H (2008) SV2A protein is a broad-spectrum anticonvulsant target: functional correlation between protein binding and seizure protection in models of both partial and generalized epilepsy. Neuropharmacology 54(4):715–720
Wandschneider B, Stretton J, Sidhu M, Centeno M, Kozak LR, Symms M, Thompson PJ, Duncan JS, Koepp MJ (2014) Levetiracetam reduces abnormal network activations in temporal lobe epilepsy. Neurology 83(17):1508–1512
Pardoe HR, Berg AT, Jackson GD (2013) Sodium valproate use is associated with reduced parietal lobe thickness and brain volume. Neurology 80(20):1895–1900
Tang Y, Yu X, Zhang X, Xia W, Wu X, Zou X, Li H, Huang X, Stefan H, Chen Q, Gong Q, Zhou D (2015) Single-dose intravenous administration of antiepileptic drugs induces rapid and reversible remodeling in the brain: Evidence from a voxel-based morphometry evaluation of valproate and levetiracetam in rhesus monkeys. Neuroscience 303:595–603
Simister RJ, McLean MA, Barker GJ, Duncan JS (2007) The effect of sodium valproate on proton MRS visible neurochemical concentrations. Epilepsy Res 74(2–3):215–219
Zeng H, Ramos CG, Nair VA, Hu Y, Liao J, La C, Chen L, Gan Y, Wen F, Hermann B, Prabhakaran V (2015) Regional homogeneity (ReHo) changes in new onset versus chronic benign epilepsy of childhood with centrotemporal spikes (BECTS): A resting state fMRI study. Epilepsy Res 116:79–85
Zhong Y, Lu G, Zhang Z, Jiao Q, Li K, Liu Y (2011) Altered regional synchronization in epileptic patients with generalized tonic-clonic seizures. Epilepsy Res 97(1–2):83–91
Zang Y, Jiang T, Lu Y, He Y, Tian L (2004) Regional homogeneity approach to fMRI data analysis. Neuroimage 22(1):394–400
Tang YL, Ji GJ, Yu Y, Wang J, Wang ZJ, Zang YF, Liao W, Ding MP (2014) Altered regional homogeneity in rolandic epilepsy: a resting-state FMRI study. BioMed Res Int 2014:960395
Mankinen K, Long XY, Paakki JJ, Harila M, Rytky S, Tervonen O, Nikkinen J, Starck T, Remes J, Rantala H, Zang YF, Kiviniemi V (2011) Alterations in regional homogeneity of baseline brain activity in pediatric temporal lobe epilepsy. Brain Res 1373:221–229
Yang T, Fang Z, Ren J, Xiao F, Li Q, Liu L, Lei D, Gong Q, Zhou D (2014) Altered spontaneous activity in treatment-naive childhood absence epilepsy revealed by Regional Homogeneity. J Neurol Sci 340(1–2):58–62
Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde BW, Engel J, French J, Glauser TA, Mathern GW, Moshe SL, Nordli D, Plouin P, Scheffer IE (2010) Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 51(4):676–685
Poline JB, Worsley KJ, Evans AC, Friston KJ (1997) Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage 5(2):83–96
Lerch JP, Worsley K, Shaw WP, Greenstein DK, Lenroot RK, Giedd J, Evans AC (2006) Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. Neuroimage 31(3):993–1003
Jokeit H, Okujava M, Woermann FG (2001) Carbamazepine reduces memory induced activation of mesial temporal lobe structures: a pharmacological fMRI-study. BMC Neurol 1:6
Szaflarski JP, Allendorfer JB (2012) Topiramate and its effect on fMRI of language in patients with right or left temporal lobe epilepsy. Epilepsy Behav 24(1):74–80
Koepp MJ (2011) Gender and drug effects on neuroimaging in epilepsy. Epilepsia 52(Suppl 4):35–37
de Saint-Martin A, Petiau C, Massa R, Maquet P, Marescaux C, Hirsch E, Metz-Lutz MN (1999) Idiopathic rolandic epilepsy with "interictal" facial myoclonia and oromotor deficit: a longitudinal EEG and PET study. Epilepsia 40(5):614–620
Archer J, Briellman R, Abbott D, Syngeniotis A, Wellard RM, Jackson G (2003) Benign Epilepsy with Centro-temporal Spikes: Spike Triggered fMRI Shows Somato-sensory CortexActivity. Epilepsia 44(2):200–204
Boor S, Vucurevic G, Pfleiderer C, Stoeter P, Kutschke G, Boor R (2003) EEG-related Functional MRI in Benign Childhood Epilepsy with CentrotemporalSpikes. Epilepsia 44(5):688–692
Boor R, Jacobs J, Hinzmann A, Bauermann T, Scherg M, Boor S, Vucurevic G, Pfleiderer C, Kutschke G, Stoeter P (2007) Combined spike-related functional MRI and multiple source analysis in the non-invasive spike localization of benign rolandic epilepsy. Clin Neurophysiol 118(4):901–909
Masterton RA, Harvey AS, Archer JS, Lillywhite LM, Abbott DF, Scheffer IE, Jackson GD (2010) Focal epileptiform spikes do not show a canonical BOLD response in patients with benign rolandic epilepsy (BECTS). Neuroimage 51(1):252–260
Norden AD, Blumenfeld H (2002) The role of subcortical structures in human epilepsy. Epilepsy Behav: E&B 3(3):219–231
Zhang ZQ, Liao W, Chen HF, Mantini D, Ding JR, Xu Q, Wang ZG, Yuan CP, Chen GH, Jiao Q, Lu GM (2011) Altered functional-structural coupling of large-scale brain networks in idiopathic generalized epilepsy. Brain: J Neurol 134(Pt 10):2912–2928
Liao W, Zhang Z, Mantini D, Xu Q, Ji GJ, Zhang H, Wang J, Wang Z, Chen G, Tian L, Jiao Q, Zang YF, Lu G (2014) Dynamical intrinsic functional architecture of the brain during absence seizures. Brain Struct Funct 219(6):2001–2015
Jang JW, Youn YC, Seok JW, Ha SY, Shin HW, Ahan SW, Park KY, Kwon OS (2011) Hypermetabolism in the left thalamus and right inferior temporal area on positron emission tomography-statistical parametric mapping (PET-SPM) in a patient with Charles Bonnet syndrome resolving after treatment with valproic acid. J Clin Neurosci: Off J Neurosurg Soc Aust 18(8):1130–1132
Gaillard WD, Zeffiro T, Fazilat S, DeCarli C, Theodore WH (1996) Effect of valproate on cerebral metabolism and blood flow: an 18F-2-deoxyglucose and 15O water positron emission tomography study. Epilepsia 37(6):515–521
Shorvon SD, Lowenthal A, Janz D, Bielen E, Loiseau P (2000) Multicenter double-blind, randomized, placebo-controlled trial of levetiracetam as add-on therapy in patients with refractory partial seizures. European Levetiracetam Study Group. Epilepsia 41(9):1179–1186
Berkovic SF, Knowlton RC, Leroy RF, Schiemann J, Falter U, Levetiracetam NSG (2007) Placebo-controlled study of levetiracetam in idiopathic generalized epilepsy. Neurology 69(18):1751–1760
Fattore C, Boniver C, Capovilla G, Cerminara C, Citterio A, Coppola G, Costa P, Darra F, Vecchi M, Perucca E (2011) A multicenter, randomized, placebo-controlled trial of levetiracetam in children and adolescents with newly diagnosed absence epilepsy. Epilepsia 52(4):802–809
Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, Fuks B (2004) The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci U S A 101(26):9861–9866
Crowder KM, Gunther JM, Jones TA, Hale BD, Zhang HZ, Peterson MR, Scheller RH, Chavkin C, Bajjalieh SM (1999) Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A). Proc Natl Acad Sci U S A 96(26):15268–15273
Crevecoeur J, Foerch P, Doupagne M, Thielen C, Vandenplas C, Moonen G, Deprez M, Rogister B (2013) Expression of SV2 isoforms during rodent brain development. BMC Neurosci 14:87
Shiraishi H, Haginoya K, Nakagawa E, Saitoh S, Kaneko Y, Nakasato N, Chan D, Otsubo H (2014) Magnetoencephalography localizing spike sources of atypical benign partial epilepsy. Brain Dev 36(1):21–27
Davis R, Peters DH, McTavish D (1994) Valproic acid. A reappraisal of its pharmacological properties and clinical efficacy in epilepsy. Drugs 47(2):332–372
Calandre EP, Dominguez-Granados R, Gomez-Rubio M, Molina-Font JA (1990) Cognitive effects of long-term treatment with phenobarbital and valproic acid in school children. Acta Neurol Scand 81(6):504–506
Umka J, Mustafa S, ElBeltagy M, Thorpe A, Latif L, Bennett G, Wigmore PM (2010) Valproic acid reduces spatial working memory and cell proliferation in the hippocampus. Neuroscience 166(1):15–22
Senturk V, Goker C, Bilgic A, Olmez S, Tugcu H, Oncu B, Atbasoglu EC (2007) Impaired verbal memory and otherwise spared cognition in remitted bipolar patients on monotherapy with lithium or valproate. Bipolar Disord 9(Suppl 1):136–144
Cysique LA, Maruff P, Brew BJ (2006) Valproic acid is associated with cognitive decline in HIV-infected individuals: a clinical observational study. BMC Neurol 6:42
Koo DL, Hwang KJ, Kim D, Kim YJ, Kim JY, Shin W, Kim MR, Joo EY, Lee JM, Hong SB (2013) Effects of levetiracetam monotherapy on the cognitive function of epilepsy patients. Eur Neurol 70(1–2):88–94
Cho JR, Koo DL, Joo EY, Yoon SM, Ju E, Lee J, Kim DY, Hong SB (2012) Effect of levetiracetam monotherapy on background EEG activity and cognition in drug-naive epilepsy patients. Clin Neurophysiol: Off J Int Fed Clin Neurophysiol 123(5):883–891
Schiemann-Delgado J, Yang H, Loge Cde L, Stalvey TJ, Jones J, Legoff D, Mintz M (2012) A long-term open-label extension study assessing cognition and behavior, tolerability, safety, and efficacy of adjunctive levetiracetam in children aged 4 to 16 years with partial-onset seizures. J Child Neurol 27(1):80–89
Acknowledgements
We thank Prof. Allen Song from Duke University and Prof. Pierre Sirois from Laval University for helpful discussions. The scientific guarantors of this publication are Guang Ming Lu and Zhi Qiang Zhang. This research was approved by the local medical ethics committee, and written informed consent was obtained from each participant. This study received funding from the Natural Science Foundation of China (Grant nos. 81422022, 81271553, 81401402, 81471653, 81201078, and 81201155), 863 project (Grant nos. 2014BAI04B05 and 2015AA020505), the Wellcome Trust (Grant no. 101253/Z/13/Z), and the China Postdoctoral Science Foundation (Grant no. 2013 M532229).
One of the authors has significant statistical expertise. Institutional Review Board approval was obtained. Study participants or cohorts have not been previously reported.
Methodology: retrospective, cross-sectional study, performed at one institution.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interests
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Presentation in part or whole at meeting: none.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 506 kb)
Rights and permissions
About this article
Cite this article
Zhang, Q., Yang, F., Hu, Z. et al. Resting-state fMRI revealed different brain activities responding to valproic acid and levetiracetam in benign epilepsy with central-temporal spikes. Eur Radiol 27, 2137–2145 (2017). https://doi.org/10.1007/s00330-016-4531-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4531-z