Abstract
Objectives
To identify the multiparametric magnetic resonance imaging (mpMRI) characteristics of normal, benign and malignant conditions in the prostate.
Methods
Fifty-six histopathological whole-mount radical prostatectomy specimens from ten randomly selected patients with prostate cancer (PC) were matched with corresponding transverse mpMRI slices. The mpMRI was performed prior to biopsy and consisted of T2-weighted imaging (T2-WI), diffusion-weighted imaging (DWI), dynamic contrast-enhanced imaging (DCE) and magnetic resonance spectroscopic imaging (MRSI).
Results
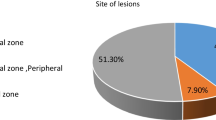
In each prostate specimen, a wide range of histopathological conditions were observed. They showed consistent but overlapping characteristics on mpMRI. Normal glands in the transition zone showed lower signal intensity (SI) on T2-WI, lower ADC values and lower citrate peaks on MRSI as compared to the peripheral zone (PZ) due to sparser glandular elements and more prominent collagenous fibres. In the PZ, normal glands were iso-intense on T2-WI, while high SI areas represented cystic atrophy. Mimickers of well-differentiated PC on mpMRI were inflammation, adenosis, HG-PIN and post-atrophic hyperplasia.
Conclusion
Each prostate is a unique mix of normal, benign and/or malignant areas that vary in extent and distribution resulting in very heterogeneous characteristics on mpMRI. Understanding the main concepts of this mpMRI-histopathological correlation may increase the diagnostic confidence in reporting mpMRI.
Keypoints
• In each prostate specimen a wide range of histopathological conditions was observed.
• Interpretation of mpMRI may be difficult because benign conditions may mimic PC.
• High signal intensity areas in the PZ on T2-WI represented cystic atrophy.
• The TZ showed sparser glands and more collagenous fibres than the PZ.






Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- BCH:
-
Basal cell hyperplasia
- CyA:
-
Cystic atrophy
- DCE:
-
Dynamic contrast-enhanced imaging
- DWI:
-
Diffusion weighted imaging
- FMH:
-
Fibromuscular hyperplasia
- FMS:
-
Fibromuscular stroma
- HG-PIN:
-
High-grade prostatic intra-epithelial neoplasia
- mpMRI:
-
Multiparametric magnetic resonance imaging
- MRSI:
-
Magnetic resonance spectroscopic imaging
- PA:
-
Partial atrophy
- PAH:
-
Post-atrophic hyperplasia
- PC:
-
Prostate cancer
- PZ:
-
Peripheral zone
- SA:
-
Simple atrophy
- SI:
-
Signal intensity
- T2-WI:
-
T2-weighted imaging
- TZ:
-
Transition zone
References
Barentsz JO, Richenberg J, Clements R et al (2012) ESUR prostate MR guidelines 2012. Eur Radiol 22:746–757
Fuchsjager M, Shukla-Dave A, Akin O, Barentsz J, Hricak H (2008) Prostate cancer imaging. Acta Radiol 49:107–120
Mazaheri Y, Shukla-Dave A, Muellner A, Hricak H (2008) MR imaging of the prostate in clinical practice. MAGMA 21:379–392
Villeirs GM, De Meerleer GO (2007) Magnetic resonance imaging (MRI) anatomy of the prostate and application of MRI in radiotherapy planning. Eur J Radiol 63:361–368
Somford DM, Futterer JJ, Hambrock T, Barentsz JO (2008) Diffusion and perfusion MR imaging of the prostate. Magn Reson Imaging Clin N Am 16(685-695):ix
Seitz M, Shukla-Dave A, Bjartell A et al (2009) Functional magnetic resonance imaging in prostate cancer. Eur Urol 55:801–814
Van As N, Charles-Edwards E, Jackson A et al (2008) Correlation of diffusion-weighted MRI with whole mount radical prostatectomy specimens. Br J Radiol 81:456–462
Coakley FV, Qayyum A, Kurhanewicz J (2003) Magnetic resonance imaging and spectroscopic imaging of prostate cancer. J Urol 170:S69–75
Klijn S, De Visschere PJ, De Meerleer GO, Villeirs GM (2012) Comparison of qualitative and quantitative approach to prostate MR spectroscopy in peripheral zone cancer detection. Eur J Radiol 81:411–416
Kurhanewicz J, Vigneron DB (2008) Advances in MR spectroscopy of the prostate. Magn Reson Imaging Clin N Am 16:697–710
Schiebler ML, Schnall MD, Pollack HM et al (1993) Current role of MR imaging in the staging of adenocarcinoma of the prostate. Radiology 189:339–352
Panebianco V, Barchetti F, Barentsz J et al (2015) Pitfalls in Interpreting mp-MRI of the Prostate: A Pictorial Review with Pathologic Correlation. Insights Imaging 6:611–630
Quon JS, Moosavi B, Khanna M, Flood TA, Lim CS, Schieda N (2015) False positive and false negative diagnoses of prostate cancer at multi-parametric prostate MRI in active surveillance. Insights Imaging. doi:10.1007/s13244-015-0411-3
De Visschere P, Oosterlinck W, De Meerleer G, Villeirs G (2010) Clinical and imaging tools in the early diagnosis of prostate cancer, a review. JBR-BTR 93:62–70
Sato C, Naganawa S, Nakamura T et al (2005) Differentiation of noncancerous tissue and cancer lesions by apparent diffusion coefficient values in transition and peripheral zones of the prostate. J Magn Reson Imaging 21:258–262
Lovett K, Rifkin MD, McCue PA, Choi H (1992) MR imaging characteristics of noncancerous lesions of the prostate. J Magn Reson Imaging 2:35–39
Hom JJ, Coakley FV, Simko JP et al (2007) High-grade prostatic intraepithelial neoplasia in patients with prostate cancer: MR and MR spectroscopic imaging features--initial experience. Radiology 242:483–489
Sciarra A, Panebianco V, Ciccariello M et al (2010) Magnetic resonance spectroscopic imaging (1H-MRSI) and dynamic contrast-enhanced magnetic resonance (DCE-MRI): pattern changes from inflammation to prostate cancer. Cancer Invest 28:424–432
Prando A, Billis A (2009) Focal prostatic atrophy: mimicry of prostatic cancer on TRUS and 3D-MRSI studies. Abdom Imaging 34:271–275
Schiebler ML, Tomaszewski JE, Bezzi M et al (1989) Prostatic carcinoma and benign prostatic hyperplasia: correlation of high-resolution MR and histopathologic findings. Radiology 172:131–137
Villeirs GM, Oosterlinck W, Vanherreweghe E, De Meerleer GO (2010) A qualitative approach to combined magnetic resonance imaging and spectroscopy in the diagnosis of prostate cancer. Eur J Radiol 73:352–356
Kurhanewicz J, Vigneron DB, Hricak H, Narayan P, Carroll P, Nelson SJ (1996) Three-dimensional H-1 MR spectroscopic imaging of the in situ human prostate with high (0.24-0.7-cm3) spatial resolution. Radiology 198:795–805
Nickel JC, True LD, Krieger JN, Berger RE, Boag AH, Young ID (2001) Consensus development of a histopathological classification system for chronic prostatic inflammation. BJU Int 87:797–805
Song L, Zhu Y, Han P et al (2011) A retrospective study: correlation of histologic inflammation in biopsy specimens of Chinese men undergoing surgery for benign prostatic hyperplasia with serum prostate-specific antigen. Urology 77:688–692
Gleason DF (1966) Classification of prostatic carcinomas. Cancer Chemother Rep 50:125–128
Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL, Committee IG (2005) The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol 29:1228–1242
De Marzo AM, Platz EA, Epstein JI et al (2006) A working group classification of focal prostate atrophy lesions. Am J Surg Pathol 30:1281–1291
Montironi R, Mazzucchelli R, Algaba F, Lopez-Beltran A (2000) Morphological identification of the patterns of prostatic intraepithelial neoplasia and their importance. J Clin Pathol 53:655–665
Montironi R, Scarpelli M, Mazzucchelli R, Cheng L, Lopez-Beltran A (2012) The spectrum of morphology in non-neoplastic prostate including cancer mimics. Histopathology 60:41–58
Epstein JI (2008) Biopsy Interpretation of the Prostate. Lippincott Williams & Wilkins, Philadelphia
McNeal JE (1988) Normal histology of the prostate. Am J Surg Pathol 12:619–633
Coakley FV, Hricak H (2000) Radiologic anatomy of the prostate gland: a clinical approach. Radiol Clin North Am 38:15–30
Weinreb JC, Barentsz JO, Choyke PL et al (2016) PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol 69:16–40
Barentsz JO, Weinreb JC, Verma S et al (2016) Synopsis of the PI-RADS v2 Guidelines for Multiparametric Prostate Magnetic Resonance Imaging and Recommendations for Use. Eur Urol 69:41–49
Hricak H, Dooms GC, McNeal JE et al (1987) MR imaging of the prostate gland: normal anatomy. AJR Am J Roentgenol 148:51–58
Langer DL, van der Kwast TH, Evans AJ et al (2008) Intermixed normal tissue within prostate cancer: effect on MR imaging measurements of apparent diffusion coefficient and T2--sparse versus dense cancers. Radiology 249:900–908
Bratan F, Niaf E, Melodelima C et al (2013) Influence of imaging and histological factors on prostate cancer detection and localisation on multiparametric MRI: a prospective study. Eur Radiol 23:2019–2029
Srigley JR (2004) Benign mimickers of prostatic adenocarcinoma. Mod Pathol 17:328–348
Nagel KN, Schouten MG, Hambrock T et al (2013) Differentiation of prostatitis and prostate cancer by using diffusion-weighted MR imaging and MR-guided biopsy at 3 T. Radiology 267:164–172
Kawada H, Kanematsu M, Goshima S et al (2015) Multiphase contrast-enhanced magnetic resonance imaging features of Bacillus Calmette-Guerin-induced granulomatous prostatitis in five patients. Korean J Radiol 16:342–348
Weinreb JC, Barentsz JO, Choyke PL et al (2015) PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol. doi:10.1016/j.eururo.2015.08.052
Rosenkrantz AB, Mendrinos S, Babb JS, Taneja SS (2012) Prostate cancer foci detected on multiparametric magnetic resonance imaging are histologically distinct from those not detected. J Urol 187:2032–2038
Villeirs GM, De Meerleer GO, De Visschere PJ, Fonteyne VH, Verbaeys AC, Oosterlinck W (2011) Combined magnetic resonance imaging and spectroscopy in the assessment of high grade prostate carcinoma in patients with elevated PSA: a single-institution experience of 356 patients. Eur J Radiol 77:340–345
Trivedi H, Turkbey B, Rastinehad AR et al (2012) Use of patient-specific MRI-based prostate mold for validation of multiparametric MRI in localization of prostate cancer. Urology 79:233–239
Acknowledgments
An abstract of this paper won the 3rd prize for best oral presentation at the 22nd European Symposium on Urogenital Radiology of the ESUR (European Society of Urogenital Radiology) annual meeting held in Copenhagen, Denmark, September 2015.
The scientific guarantor of this publication is Geert Villeirs. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was not required for this study because it was a retrospective study. Written informed consent was waived by the Institutional Review Board. Methodology: retrospective, observational, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Additional information
Pieter J. L. De Visschere and Anne Vral contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure 1
Photograph of a macroslide (Patient 1) showing how the different histological conditions were delineated, based on the observations on the high magnification views. This scheme was used for evaluating the imaging characteristics of the different histological conditions on the corresponding T2-WI, DWI, DCE and MRSI images of the mpMRI. (GIF 10.2 MB)
Supplemental Figure 2
prostate glands (Patient 4, 12.5x), B. Basal cell hyperplasia (Patient 4, 12.5x), C. Simple atrophy (Patient 4, 12.5x), D. Partial atrophy (Patient 4, 12.5x), E.Post-atrophic hyperplasia (Patient 4, 3.2x), F. Cystic atrophy (Patient 10, 3.2x). (GIF 11.4 MB)
Supplemental Figure 3
High magnification histopathological images of PC, HG-PIN and benign mimickers of PC. A. Poorly-differentiated PC (Patient 10, 25x), B. Well-differentiated PC (Patient 7, 25x), C. HG-PIN, tufted phenotype (Patient 2, 25x), D. Adenosis (Patient 2, 3.2x), E. Severe stromal and periglandular inflammation (Patient 5, 3.2x), F. Well-defined, pale-stained nodule of fibromuscular hyperplasia embedded within a peri-urethral area of stroma (Patient 2, 3.2x). (GIF 3.68 MB)
Supplemental Figure 4
A. Photograph of macroslide, B. T2-WI, C. ADC map of DWI, D. subtraction image of DCE, E. MRSI of CyA, F. MRSI of SA (Patient 3). Cystic atrophy (dashed line) and simple atrophy (dotted line) both show a high SI on T2-WI (B) and high ADC values on DWI (C). SA is however ill defined and lacks the black rims of FMS that are typical for CyA (B). CyA shows no contrast enhancement whereas simple atrophy shows minimal contrast enhancement (D). On MRSI both show high citrate peaks and low choline peaks, but in CyA (E) the citrate peaks are lower than in SA (F). (GIF 3.43 MB)
Supplemental Figure 5
A. Photograph of macroslide, B. T2-WI, C. ADC map of DWI, D. subtraction image of DCE, E. MRSI map, F. MRSI of FMH, G. MRSI of mixture of simple and cystic atrophy (Patient 3). Fibromuscular hyperplasia (dotted line) is a common finding in the TZ and appears as a very hypointense round nodule on T2-WI (B). On DWI it has a low ADC value (C) and on DCE it shows moderate contrast enhancement (D). On MRSI it demonstrates low peaks of all metabolites (G). There is a big contrast with a mixture of simple atrophy and cystic atrophy (dashed line) that shows a moderately high SI on T2-WI (B), high ADC values on DWI (C), low contrast enhancement on DCE (D) and high citrate peaks on MRSI (E, G). (GIF 4.40 MB)
Supplemental Figure 6
A. Photograph of macroslide, B. T2-WI, C. ADC map of DWI, D. subtraction image of DCE, E. MRSI (Patient 2) Post-atrophic hyperplasia (black line) is usually tiny or very small in size but in this patient a larger nodule was observed histologically, large enough to correlate with the corresponding axial slices of mpMRI, but it showed no specific features: it was isointense on T2-WI (B), showed no restricted diffusion (C) and minimal contrast enhancement (D). Cystic atrophy (dashed line) could be recognized easily in this patient as a kidney-shaped lesion with very high SI on T2-WI (B), high ADC value on DWI (C) and absent contrast enhancement (D). Poorly differentiated PC (white line) in the TZ is demonstrated as an ill-defined irregular homogeneous hypointense lesion on T2-WI (B), with low ADC value on DWI (C) and strong contrast enhancement (D). On MRSI the citrate peaks are decreased (E). Fibromuscular stroma (dotted line) also has low SI on T2-WI (B), with low ADC value on DWI (C) but with fewer contrast enhancement as compared to poorly differentiated PC (D). (GIF 3.21 MB)
Supplemental Figure 7
A. Photograph of macroslide, B. T2-WI, C. ADC map of DWI, D. high b-value image of DWI, E. subtraction image of DCE, F. DCE enhancement curve of poorly differentiated PC, G. DCE enhancement curve of cystic atrophy, I. MRSI (Patient 3). Poorly differentiated PC (dotted line) is demonstrated as a very low SI nodule on T2-WI (B) with restricted diffusion (C, D) and strong contrast enhancement (E) with high initial contrast peak and wash out shape of the DCE curve (F). On MRSI the citrate peaks are low (I). Cystic atrophy (dashed line) and simple atrophy (white line) show high SI on T2-WI (B) with on DWI high ADC value (C) and low signal on high-b-value image (D). They show absent or minimal contrast enhancement (E) with linear shape of the enhancement curve of DCE (G). On MRSI the citrate peaks are moderately high (G). (JPEG 708 kb)
Rights and permissions
About this article
Cite this article
De Visschere, P.J.L., Vral, A., Perletti, G. et al. Multiparametric magnetic resonance imaging characteristics of normal, benign and malignant conditions in the prostate. Eur Radiol 27, 2095–2109 (2017). https://doi.org/10.1007/s00330-016-4479-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4479-z