Abstract
Objectives
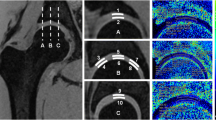
To investigate the influence of intravenous gadolinium on cartilage T2 and T2* relaxation times and on morphological image quality at 7-T hip MRI.
Methods
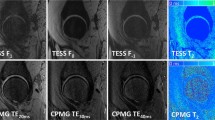
Hips of 11 healthy volunteers were examined at 7 T. Multi-echo sequences for T2 and T2* mapping, 3D T1 volumetric interpolated breath-hold examination (VIBE) and double-echo steady-state (DESS) sequences were acquired before and after intravenous application of gadolinium according to a delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) protocol. Cartilage relaxation times were measured in both scans. Morphological sequences were assessed quantitatively using contrast ratios and qualitatively using a 4-point Likert scale. Student’s t-test, Pearson’s correlation (ρ) and Wilcoxon sign-rank test were used for statistical comparisons.
Results
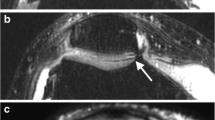
Pre- and post-contrast T2 and T2* values were highly correlated (T2: acetabular: ρ = 0.76, femoral: ρ = 0.77; T2*: acetabular: ρ = 0.80, femoral: ρ = 0.72). Gadolinium enhanced contrasts between cartilage and joint fluid in DESS and T1 VIBE according to the qualitative (p = 0.01) and quantitative (p < 0.001) analysis. The delineation of acetabular and femoral cartilage and the labrum predominantly improved with gadolinium.
Conclusions
Gadolinium showed no relevant influence on T2 or T2* relaxation times and improved morphological image quality at 7 T. Therefore, morphological and quantitative sequences including dGEMRIC can be conducted in a one-stop-shop examination.
Key Points
• Hip cartilage T2 values correlate highly before and after gadolinium at 7 T
• Hip cartilage T2* values correlate highly before and after enhancement at 7 T
• Morphological hip cartilage imaging benefits from intravenous gadolinium at 7 T
• The delineation of acetabular and femoral cartilage can be improved by gadolinium
• Morphological and quantitative sequences including dGEMRIC can be combined as a one-stop-shop examination





Similar content being viewed by others
Abbreviations
- MRI:
-
Magnetic resonance imaging
- T:
-
Tesla
- SD:
-
Standard deviation
- BMI:
-
Body mass index
- RF:
-
Radio frequency
- FLASH:
-
Fast low-angle shot
- DREAM:
-
Dual refocusing echo acquisition mode
- FOV:
-
Field of view
- TE:
-
Echo time
- TR:
-
Repetition time
- DESS:
-
Double-echo steady state
- VIBE:
-
Volumetric interpolated breath-hold examination
- dGEMRIC:
-
Delayed gadolinium-enhanced MRI of cartilage
- ROI:
-
Region of interest
- CR:
-
Contrast ratio
- SSFP:
-
Steady-state free precession
- FISP:
-
Fast imaging steady precession
References
Binks DA, Hodgson RJ, Ries ME et al (2013) Quantitative parametric MRI of articular cartilage: a review of progress and open challenges. Br J Radiol 86:20120163
Rogers AD, Payne JE, Yu JS (2013) Cartilage imaging: a review of current concepts and emerging technologies. Semin Roentgenol 48:148–157
Bashir A, Gray ML, Burstein D (1996) Gd-DTPA2- as a measure of cartilage degradation. Magn Reson Med 36:665–673
Liess C, Lusse S, Karger N, Heller M, Gluer CC (2002) Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthr Cartil 10:907–913
Gold SL, Burge AJ, Potter HG (2012) MRI of hip cartilage: joint morphology, structure, and composition. Clin Orthop Relat Res 470:3321–3331
Nieminen MT, Nissi MJ, Mattila L, Kiviranta I (2012) Evaluation of chondral repair using quantitative MRI. J Magn Reson Imaging 36:1287–1299
Burstein D, Velyvis J, Scott KT et al (2001) Protocol issues for delayed Gd(DTPA)(2-)-enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med 45:36–41
Morrison WB (2005) Indirect MR arthrography: concepts and controversies. Semin Musculoskelet Radiol 9:125–134
Petchprapa CN, Rybak LD, Dunham KS, Lattanzi R, Recht MP (2015) Labral and cartilage abnormalities in young patients with hip pain: accuracy of 3-Tesla indirect MR arthrography. Skelet Radiol 44:97–105
Kijowski R (2010) Clinical cartilage imaging of the knee and hip joints. AJR Am J Roentgenol 195:618–628
Krug R, Stehling C, Kelley DA, Majumdar S, Link TM (2009) Imaging of the musculoskeletal system in vivo using ultra-high field magnetic resonance at 7 T. Investig Radiol 44:613–618
Chang G, Deniz CM, Honig S et al (2014) MRI of the hip at 7T: feasibility of bone microarchitecture, high-resolution cartilage, and clinical imaging. J Magn Reson Imaging 39:1384–1393
Ellermann J, Goerke U, Morgan P et al (2012) Simultaneous bilateral hip joint imaging at 7 Tesla using fast transmit B(1) shimming methods and multichannel transmission - a feasibility study. NMR Biomed 25:1202–1208
Theysohn JM, Kraff O, Orzada S et al (2013) Bilateral hip imaging at 7 Tesla using a multi-channel transmit technology: initial results presenting anatomical detail in healthy volunteers and pathological changes in patients with avascular necrosis of the femoral head. Skelet Radiol 42:1555–1563
Theysohn JM, Kraff O, Theysohn N et al (2014) Hip imaging of avascular necrosis at 7 Tesla compared with 3 Tesla. Skelet Radiol 43:623–632
Lazik A, Theysohn JM, Geis C et al (2015) 7 Tesla quantitative hip MRI: T1, T2 and T2* mapping of hip cartilage in healthy volunteers. Eur Radiol. doi:10.1007/s00330-015-3964-0
Nissi MJ, Mortazavi S, Hughes J, Morgan P, Ellermann J (2015) T2* relaxation time of acetabular and femoral cartilage with and without intraarticular gadopentetate dimeglumine in patients with femoroacetabular impingement. AJR Am J Roentgenol 204:W695–W700
Verschueren J, Tiel J, Reijman M et al (2014) T2 relaxation times of knee articular cartilage in osteoarthritis patients are not influenced by gadolinium contrast agent. Radiological Society of North America 2014 Scientific Assembly and Annual Meeting, Chicago
Fries P, Morelli JN, Lux F, Tillement O, Schneider G, Buecker A (2014) The issues and tentative solutions for contrast-enhanced magnetic resonance imaging at ultra-high field strength. Wiley Interdiscip Rev Nanomed Nanobiotechnol 6:559–573
Kalavagunta C, Michaeli S, Metzger GJ (2014) In vitro Gd-DTPA relaxometry studies in oxygenated venous human blood and aqueous solution at 3 and 7 T. Contrast Media Mol Imaging 9:169–176
Noebauer-Huhmann IM, Szomolanyi P, Juras V, Kraff O, Ladd ME, Trattnig S (2010) Gadolinium-based magnetic resonance contrast agents at 7 Tesla: in vitro T1 relaxivities in human blood plasma. Investig Radiol 45:554–558
Orzada S, Quick HH, Ladd ME et al (2009) A flexible 8-channel transmit/receive body coil for 7 T human imaging. Proc Intl Soc Mag Reson Med 17, Hawaii, USA, pp 2999
Nehrke K, Bornert P (2012) DREAM—a novel approach for robust, ultrafast, multislice B(1) mapping. Magn Reson Med 68:1517–1526
Kraff O, Lazik A, Brenner D et al (2015) In vivo comparison of B1 mapping techniques for hip joint imaging at 7 Tesla. Proc Intl Soc Mag Reson Med 23, Toronto, Canada
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Mortazavi S, Nissi M, Hughes J, Morgan P, Ellermann J (2014) T2* relaxation time of acetabular and femoral cartilage with and without intra-articular Gd-DTPA2- in hip FAI patients. Radiological Society of North America 2014 Scientific Assembly and Annual Meeting, Chicago
Nieminen MT, Menezes NM, Williams A, Burstein D (2004) T2 of articular cartilage in the presence of Gd-DTPA2. Magn Reson Med 51:1147–1152
Kurkijarvi JE, Nissi MJ, Rieppo J et al (2008) The zonal architecture of human articular cartilage described by T2 relaxation time in the presence of Gd-DTPA2. Magn Reson Imaging 26:602–607
May DA, Pennington DJ (2000) Effect of gadolinium concentration on renal signal intensity: An in vitro study with a saline bag model. Radiology 216:232–236
Sutter R, Zubler V, Hoffmann A et al (2014) Hip MRI: how useful is intraarticular contrast material for evaluating surgically proven lesions of the labrum and articular cartilage? AJR Am J Roentgenol 202:160–169
Vahlensieck M, Peterfy CG, Wischer T et al (1996) Indirect MR arthrography: optimization and clinical applications. Radiology 200:249–254
Vahlensieck M, Sommer T, Textor J et al (1998) Indirect MR arthrography: techniques and applications. Eur Radiol 8:232–235
Peterfy CG, Schneider E, Nevitt M (2008) The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthr Cartil 16:1433–1441
Lee MJ, Motamedi K, Chow K, Seeger LL (2008) Gradient-recalled echo sequences in direct shoulder MR arthrography for evaluating the labrum. Skelet Radiol 37:19–25
Schmitt R, Christopoulos G, Meier R et al (2003) Direct MR arthrography of the wrist in comparison with arthroscopy: a prospective study on 125 patients. Röfo 175:911–919
Juras V, Bohndorf K, Heule R et al (2015) A comparison of multi-echo spin-echo and triple-echo steady-state T2 mapping for in vivo evaluation of articular cartilage. Eur Radiol. doi:10.1007/s00330-015-3979-6
Paunipagar BK, Rasalkar D (2014) Imaging of articular cartilage. Indian J Radiol Imaging 24:237–248
Roemer FW, Kwoh CK, Hannon MJ et al (2011) Semiquantitative assessment of focal cartilage damage at 3T MRI: a comparative study of dual echo at steady state (DESS) and intermediate-weighted (IW) fat suppressed fast spin echo sequences. Eur J Radiol 80:e126–e131
Schmitt B, Zbyn S, Stelzeneder D et al (2011) Cartilage quality assessment by using glycosaminoglycan chemical exchange saturation transfer and (23)Na MR imaging at 7 T. Radiology 260:257–264
Raya JG, Dettmann E, Notohamiprodjo M, Krasnokutsky S, Abramson S, Glaser C (2014) Feasibility of in vivo diffusion tensor imaging of articular cartilage with coverage of all cartilage regions. Eur Radiol 24:1700–1706
Zbyn S, Mlynarik V, Juras V, Szomolanyi P, Trattnig S (2015) Evaluation of cartilage repair and osteoarthritis with sodium MRI. NMR Biomed. doi:10.1002/nbm.3280
Rehnitz C, Kupfer J, Streich NA et al (2014) Comparison of biochemical cartilage imaging techniques at 3 T MRI. Osteoarthr Cartil 22:1732–1742
Acknowledgments
The authors thank Desmond Tse (Maastricht University, The Netherlands) for providing the source code of the DREAM sequence.
This work was supported by a research grant (“IFORES”) from the University Duisburg-Essen, Germany, awarded to the first author. Different results of the same study population have already been published in “7 Tesla quantitative hip MRI: T1, T2 and T2* mapping of hip cartilage in healthy volunteers” (Lazik A et al., Eur Radiol. 2015 Aug 28. DOI 10.1007/s00330-015-3964-0).
The scientific guarantor of this publication is Dr. med. Andrea Lazik-Palm. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. No complex statistical methods were necessary for this paper.
Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Methodology: prospective, experimental, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lazik-Palm, A., Kraff, O., Geis, C. et al. Morphological imaging and T2 and T2* mapping of hip cartilage at 7 Tesla MRI under the influence of intravenous gadolinium. Eur Radiol 26, 3923–3931 (2016). https://doi.org/10.1007/s00330-016-4247-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4247-0