Abstract
Objective
We compared the precentral gyri (PG) on the PADRE of patients with amyotrophic lateral sclerosis (ALS) and healthy subjects (HSs) in order to determine whether it is possible to discriminate between ALS patients and HSs on an individual basis.
Methods
First, two radiologists reviewed the appearance of the normal PG and that of ALS patients on PADRE in a non-blinded manner, and deviations from the appearance of the normal PG were recorded. Next, based on the presence of PG abnormalities on PADRE, we performed an observer performance study using 16 ALS patients and 16 HSs.
Results
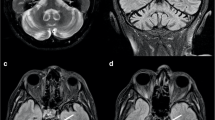
The radiologists were able to consensually define the PG as abnormal on PADRE when a low-signal-intensity layer was observed in the gray matter of the PG; a three- or four-layer organization (zebra sign) was characterized by the low-signal-intensity layer. The observer performance study demonstrated that the sensitivity, specificity, and accuracy of PG abnormalities on PADRE for discriminating ALS patients from HSs were 94 %, 94 %, and 94 %, respectively, for reviewers 1 and 2.
Conclusions
It was possible to discriminate between ALS patients and HSs based on the presence of PG abnormalities on PADRE, which may reflect upper motor neuron impairment in ALS.
Key Points
• PADRE reveals low-signal-intensity layer in the PG of ALS
• By PADRE findings on PG, we can discriminate ALS from HSs
• PADRE may be a useful method for detecting UMN impairment in ALS





Similar content being viewed by others
Abbreviations
- PADRE:
-
Phase difference enhanced imaging
- SWI:
-
Susceptibility-weighted imaging
- ALS:
-
Amyotrophic lateral sclerosis
- PLS:
-
Primary lateral sclerosis
- GM:
-
Gray matter
- WM:
-
White matter
- LSIL:
-
Low-signal-intensity layer
- UMN:
-
Upper motor neuron
- LMN:
-
Lower motor neuron
References
Urban PP, Wicht S, Hopf H (2001) Sensitivity of transcranial magnetic stimulation of cortico-bulbar vs. cortico-spinal tract involvement in Amyotrophic Lateral Sclerosis (ALS). J Neurol 248:850–855
Zinman L, Cudkowicz M (2011) Emerging targets and treatments in amyotrophic lateral sclerosis. Lancet Neurol 10:481–490
Zhang L, Uluǧ AM, Zimmerman RD, Lin MT, Rubin M, Beal MF (2003) The diagnostic utility of FLAIR imaging in clinically verified amyotrophic lateral sclerosis. J Magn Reson Imaging 17:521–527
Kamada K, Kakeda S, Ohnari N, Moriya J, Sato T, Korogi Y (2008) Signal intensity of motor and sensory cortices on T2-weighted and FLAIR images: intraindividual comparison of 1.5 T and 3T MRI. Eur Radiol 18:2949–2955
Bowen BC, Pattany PM, Bradley WG et al (2000) MR imaging and localized proton spectroscopy of the precentral gyrus in amyotrophic lateral sclerosis. Am J Neuroradiol 21:647–658
Graham J, Papadakis N, Evans J et al (2004) Diffusion tensor imaging for the assessment of upper motor neuron integrity in ALS. Neurology 63:2111–2119
Sach M, Winkler G, Glauche V et al (2004) Diffusion tensor MRI of early upper motor neuron involvement in amyotrophic lateral sclerosis. Brain 127:340–350
Weisstanner C, Gratz PP, Schroth G et al (2014) Thrombus imaging in acute stroke: correlation of thrombus length on susceptibility-weighted imaging with endovascular reperfusion success. Eur Radiol 24:1735–1741
Fu J-H, Chuang T-C, Chung H-W et al (2015) Discriminating pyogenic brain abscesses, necrotic glioblastomas, and necrotic metastatic brain tumors by means of susceptibility-weighted imaging. Eur Radiol 25:1413–1420
Ogasawara A, Kakeda S, Watanabe K et al (2015) Quantitative susceptibility mapping in patients with systemic lupus erythematosus: detection of abnormalities in normal-appearing basal ganglia. Eur Radiol :1–8
Ide S, Kakeda S, Ueda I et al (2015) Internal structures of the globus pallidus in patients with Parkinson’s disease: evaluation with quantitative susceptibility mapping (QSM). Eur Radiol 25:710–718
Haacke EM, Ayaz M, Khan A et al (2007) Establishing a baseline phase behavior in magnetic resonance imaging to determine normal vs. abnormal iron content in the brain. J Magn Reson Imaging 26:256–264
Haacke EM, Cheng NY, House MJ et al (2005) Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging 23:1–25
Haacke EM, Xu Y, Cheng YCN, Reichenbach JR (2004) Susceptibility weighted imaging (SWI). Magn Reson Med 52:612–618
Kakeda S, Korogi Y, Yoneda T et al (2011) A novel tract imaging technique of the brainstem using phase difference enhanced imaging: normal anatomy and initial experience in multiple system atrophy. Eur Radiol 21:2202–2210
Ide S, Kakeda S, Korogi Y et al (2012) Delineation of optic radiation and stria of gennari on high-resolution phase difference enhanced imaging. Acad Radiol 19:1283–1289
Kakeda S, Korogi Y, Yoneda T et al (2013) Parkinson’s disease: diagnostic potential of high-resolution phase difference enhanced MR imaging at 3 T. Eur Radiol 23:1102–1111
Brooks BR, Miller RG, Swash M, Munsat TL (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler 1:293–299
de Carvalho M, Dengler R, Eisen A et al (2008) Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol 119:497–503
Gordon P, Cheng B, Katz I et al (2006) The natural history of primary lateral sclerosis. Neurology 66:647–653
Balendra R, Jones A, Jivraj N et al (2014) Use of clinical staging in amyotrophic lateral sclerosis for phase 3 clinical trials. J Neurol Neurosurg Psychiatry :jnnp-2013-306865
Roche JC, Rojas-Garcia R, Scott KM et al (2012) A proposed staging system for amyotrophic lateral sclerosis. Brain 135:847–852
Yousry T, Schmid U, Alkadhi H et al (1997) Localization of the motor hand area to a knob on the precentral gyrus. Brain 120:141–157
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Lambert E, Mulder D (1957) Electromyographic studies in amyotrophic lateral sclerosis. Proceedings of the staff meetings Mayo Clinic, pp 441
Kakeda S, Korogi Y, Kamada K et al (2008) Signal intensity of the motor cortex on phase-weighted imaging at 3T. Am J Neuroradiol 29:1171–1175
Tong KA, Ashwal S, Holshouser BA et al (2003) Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury: improved detection and initial results 1. Radiology 227:332–339
Kakeda S, Yoneda T, Ide S, Watanabe K, Hiai Y, Korogi Y (2015) Signal intensity of superficial white matter on phase difference enhanced imaging as a landmark of the perirolandic cortex. Acta Radiol :0284185115585162
Jones E, Coulter J, Wise S (1979) Commissural columns in the sensory motor cortex of monkeys. J Comp Neurol 188:113–135
Udaka F, Kameyama M, Tomonaga M (1986) Degeneration of Betz cells in motor neuron disease. A Golgi study. Acta Neuropathol 70:289–295
Kwan JY, Jeong SY, Van Gelderen P et al (2012) Iron accumulation in deep cortical layers accounts for MRI signal abnormalities in ALS: correlating 7 Tesla MRI and pathology. PLoS One 7, e35241
Yu J, Qi F, Wang N et al (2014) Increased iron level in motor cortex of amyotrophic lateral sclerosis patients: an in vivo MR study. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration :1–5
Schweitzer AD, Liu T, Gupta A et al (2015) Quantitative susceptibility mapping of the motor cortex in amyotrophic lateral sclerosis and primary lateral sclerosis. Am J Roentgenol 204:1086–1092
Acknowledgements
The scientific guarantor of this publication is Yukunori Korogi, M.D. The authors of this manuscript declare no relationships with any companies. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was waived by the Institutional Review Board. Methodology: retrospective case-control study performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kakeda, S., Yoneda, T., Ide, S. et al. Zebra sign of precentral gyri in amyotrophic lateral sclerosis: A novel finding using phase difference enhanced (PADRE) imaging-initial results. Eur Radiol 26, 4173–4183 (2016). https://doi.org/10.1007/s00330-016-4219-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4219-4