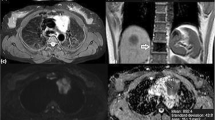
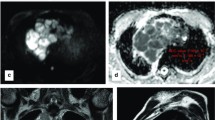
Abstract
Objectives
To evaluate the usefulness of diffusion-weighted magnetic resonance for distinguishing thymomas according to WHO and Masaoka-Koga classifications and in predicting disease-free survival (DFS) by using the apparent diffusion coefficient (ADC).
Methods
Forty-one patients were grouped based on WHO (low-risk vs. high-risk) and Masaoka-Koga (early vs. advanced) classifications. For prognosis, seven patients with recurrence at follow-up were grouped separately from healthy subjects. Differences on ADC levels between groups were tested using Student-t testing. Logistic regression models and areas under the ROC curve (AUROC) were estimated.
Results
Mean ADC values were different between groups of WHO (low-risk = 1.58 ± 0.20 × 10-3mm2/sec; high-risk = 1.21 ± 0.23 × 10-3mm2/sec; p < 0.0001) and Masaoka-Koga (early = 1.43 ± 0.26 × 10-3mm2/sec; advanced = 1.31 ± 0.31 × 10-3mm2/sec; p = 0.016) classifications. Mean ADC of type-B3 (1.05 ± 0.17 × 10-3mm2/sec) was lower than type-B2 (1.32 ± 0.20 × 10-3mm2/sec; p = 0.023). AUROC in discriminating groups was 0.864 for WHO classification (cut-point = 1.309 × 10-3mm2/sec; accuracy = 78.1 %) and 0.730 for Masaoka-Koga classification (cut-point = 1.243 × 10-3mm2/sec; accuracy = 73.2 %). Logistic regression models and two-way ANOVA were significant for WHO classification (odds ratio[OR] = 0.93, p = 0.007; p < 0.001), but not for Masaoka-Koga classification (OR = 0.98, p = 0.31; p = 0.38). ADC levels were significantly associated with DFS recurrence rate being higher for patients with ADC ≤ 1.299 × 10-3mm2/sec (p = 0.001; AUROC, 0.834; accuracy = 78.0 %).
Conclusions
ADC helps to differentiate high-risk from low-risk thymomas and discriminates the more aggressive type-B3. Primary tumour ADC is a prognostic indicator of recurrence.
Key Points
• DW-MRI is useful in characterizing thymomas and in predicting disease-free survival.
• ADC can differentiate low-risk from high-risk thymomas based on different histological composition
• The cutoff-ADC-value of 1.309 × 10 -3 mm 2 /sec is proposed as optimal cut-point for this differentiation
• The ADC ability in predicting Masaoka-Koga stage is uncertain and needs further validations
• ADC has prognostic value on disease-free survival and helps in stratification of risk







Similar content being viewed by others
References
Priola AM, Priola SM, Cardinale L, Cataldi A, Fava C (2006) The anterior mediastinum: diseases. Radiol Med 111:312–342
Wright CD (2008) Management of thymomas. Crit Rev Oncol Hematol 65:109–120
Nishino M, Ashiku SK, Kocher ON, Thurer RL, Boiselle PM, Hatabu H (2006) The thymus: a comprehensive review. Radiographics 26:335–348
Henschke CI, Lee IJ, Wu N et al (2006) CT screening for lung cancer: prevalence and incidence of mediastinal masses. Radiology 239:586–590
Novello S, Fava C, Borasio P et al (2005) Three-year findings of an early lung cancer detection feasibility study with low-dose spiral computed tomography in heavy smokers. Ann Oncol 16:1662–1666
Priola AM, Priola SM, Giaj-Levra M et al (2013) Clinical implications and added costs of incidental findings in an early detection study of lung cancer by using low-dose spiral computed tomography. Clin Lung Cancer 14:139–148
Müller-Hermelink HK, Ströbel P, Zettl A et al (2004) Combined thymic epithelial tumours. In: Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC (eds) Pathology and genetics of tumours of the lung, pleura, thymus and heart (WHO classification of tumours series). IARC Press, Lyon, pp 196–198
Masaoka A, Monden Y, Nakahara K, Tanioka T (1981) Follow-up study of thymomas with special reference to their clinical stages. Cancer 48:2485–2492
Jeong YJ, Lee KS, Kim J, Shim YM, Han J, Kwon OJ (2004) Does CT of thymic epithelial tumors enable us to differentiate histologic subtypes and predict prognosis? AJR Am J Roentgenol 183:283–289
Okuma Y, Hosomi Y, Watanabe K et al (2014) Clinicopathological analysis of thymic malignancies with a consistent retrospective database in a single institution: from Tokyo Metropolitan Cancer Center. BMC Cancer 14:349–357
Priola AM, Priola SM (2014) Imaging of thymus in myasthenia gravis: from thymic hyperplasia to thymic tumor. Clin Radiol 69:e230–e245
Landwehr P, Schulte O, Lackner K (1999) MR imaging of the chest: mediastinum and chest wall. Eur Radiol 9:1737–1744
Priola SM, Priola AM, Cardinale L, Perotto F, Fava C (2006) The anterior mediastinum: anatomy and imaging procedures. Radiol Med 111:295–311
Shin KE, Yi CA, Kim TS et al (2014) Diffusion-weighted MRI for distinguishing non-neoplastic cysts from solid masses in the mediastinum: problem-solving in mediastinal masses of indeterminate internal characteristics on CT. Eur Radiol 24:677–684
Gümüștaș S, Inan N, Sarisoy HT (2011) Malignant versus benign mediastinal lesions: quantitative assessment with diffusion weighted MR imaging. Eur Radiol 21:2255–2260
Abdel Razek A, Soliman N, Elashery R (2012) Apparent diffusion coefficient values of mediastinal masses in children. Eur J Radiol 81:1311–1314
Abdel Razek A, Elmorsy A, Elshafey M, Elhadedy T, Hamza O (2009) Assessment of mediastinal tumors with diffusion weighted single shot echo planar MR imaging. J Magn Reson Imaging 30:535–540
Seki S, Koyama H, Ohno Y et al (2014) Diffusion-weighted MR imaging vs. multi-detector row-CT: direct comparison of capability for assessment of management needs for anterior mediastinal solitary tumors. Eur J Radiol 83:835–842
Abdel Razek AA, Khairy M, Nada N (2015) Diffusion-weighted MR imaging in thymic epithelial tumors: correlation with World Health Organization classification and clinical staging. Radiology 273:268–275
Priola AM, Priola SM (2015) Usefulness of diffusion-weighted MR imaging in predicting Masaoka-Koga clinical staging of thymic epithelial tumors by using the apparent diffusion coefficient. Radiology 274:936–937
Filli L, Wurnig MC, Luechinger R, Eberhardt C, Guggenberger R, Boss A (2015) Whole-body intravoxel incoherent motion imaging. Eur Radiol. doi:10.1007/s00330-014-3577-z
Zhang YD, Wang Q, Wu CJ (2015) The histogram analysis of diffusion-weighted intravoxel incoherent motion (IVIM) imaging for differentiating the gleason grade of prostate cancer. Eur Radiol 25:994–1004
Padhani AR, Guoying L, Mu-Koh D et al (2009) Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 11:102–125
Koh DM, Collins DJ (2007) Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol 188:1622–1635
Priola AM, Priola SM (2013) Influence of selected b values on ADC quantification in diffusion-weighted MRI. Comment on Punwani et al.: Diffusion-weighted MRI of lymphoma: prognostic utility and implications for PET/MRI? Eur J Nucl Med Mol Imaging 40:1108–1809
Priola AM, Priola SM, Giraudo MT et al (2015) Chemical-shift and diffusion-weighted magnetic resonance imaging of thymus in myasthenia gravis: usefulness of quantitative assessment. Investig Radiol 50:228–238
Priola AM, Priola SM, Ciccone G et al (2015) Differentiation of rebound and lymphoid thymic hyperplasia from anterior mediastinal tumors with dual-echo chemical-shift magnetic resonance imaging in adulthood: reliability of the chemical-shift ratio and signal-intensity index. Radiology 274:238–249
Inchingolo R, De Gaetano AM, Curione D et al (2015) Role of diffusion-weighted imaging, apparent diffusion coefficient and correlation with hepatobiliary phase findings in the differentiation of hepatocellular carcinoma from dysplastic nodules in cirrhotic liver. Eur Radiol 25:1087–1096
Fischer MA, Nanz D, Hany T et al (2011) Diagnostic accuracy of whole-body MRI/DWI image fusion for detection of malignant tumours: a comparison with PET/CT. Eur Radiol 21:246–255
Priola AM, Galetto G, Priola SM (2014) Diagnostic and functional imaging of thymic and mediastinal involvement in lymphoproliferative disorders. Clin Imaging 38:771–784
Rieker RJ, Hoegel J, Morresi-Hauf A et al (2002) Histologic classification of thymic epithelial tumors: comparison of established classification schemes. Int J Cancer 98:900–906
Schmidt H, Gatidis S, Schwenzer NF, Martirosian P (2015) Impact of measurement parameters on apparent diffusion coefficient quantification in diffusion-weighted-magnetic resonance imaging. Investig Radiol 50:46–56
Ackman JB (2014) A practical guide to nonvascular thoracic magnetic resonance imaging. J Thorac Imaging 29:17–29
Abdel Razek AAK (2012) Diffusion magnetic resonance imaging of chest tumors. Cancer Imaging 12:452–463
Padhani AR, Koh DM, Collins DJ (2011) Whole-body diffusion-weighted MR imaging in cancer: current status and research directions. Radiology 261:700–718
Roden AC, Yi ES, Cassivi SD, Jenkins SM, Garces YI, Aubry MC (2013) Clinicopathological features of thymic carcinomas and the impact of histopathological agreement on prognostical studies. Eur J Cardiothorac Surg 43:1131–1139
Hatakenaka M, Nakamura K, Yabuuchi U et al (2014) Apparent diffusion coefficient is a prognostic factor of head and neck squamous cell carcinoma treated with radiotherapy. Jpn J Radiol 32:80–89
Giganti F, Orsenigo E, Esposito A et al (2015) Prognostic role of diffusion-weighted MR imaging for resectable gastric cancer. Radiology. doi:10.1148/radiol.15141900
Nakamura K, Joja I, Nagasaka T et al (2012) The mean apparent diffusion coefficient value (ADCmean) on primary cervical cancer is a predictive marker for disease recurrence. Gynecol Oncol 127:478–483
Seki N, Sakamoto S, Karube Y, Oyaizu T, Ishihama H, Chida M (2014) 18F-fluorodeoxyglucose positron emission tomography for evaluation of thymic epithelial tumors: utility for World Health Organization classification and predicting recurrence-free survival. Ann Nucl Med 28:257–262
Kaira K, Murakami H, Miura S et al (2011) 18F-FDG uptake on PET helps predict outcome and response after treatment in unresectable thymic epithelial tumors. Ann Nucl Med 25:247–253
Acknowledgments
The scientific guarantor of this publication is Adriano M. Priola. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. One of the authors (Maria T. Giraudo) has significant statistical expertise. Institutional review board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Methodology: prospective, observational/diagnostic or prognostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Priola, A.M., Priola, S.M., Giraudo, M.T. et al. Diffusion-weighted magnetic resonance imaging of thymoma: ability of the Apparent Diffusion Coefficient in predicting the World Health Organization (WHO) classification and the Masaoka-Koga staging system and its prognostic significance on disease-free survival. Eur Radiol 26, 2126–2138 (2016). https://doi.org/10.1007/s00330-015-4031-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-4031-6