Abstract
Objectives
To explore the role of diffusion tensor imaging (DTI)-based histogram analysis and functional diffusion maps (fDMs) in evaluating structural changes of low-grade gliomas (LGGs) receiving temozolomide (TMZ) chemotherapy.
Methods
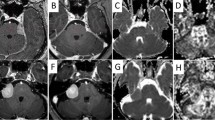
Twenty-one LGG patients underwent 3T-MR examinations before and after three and six cycles of dose-dense TMZ, including 3D-fluid-attenuated inversion recovery (FLAIR) sequences and DTI (b = 1000 s/mm2, 32 directions). Mean diffusivity (MD), fractional anisotropy (FA), and tensor-decomposition DTI maps (p and q) were obtained. Histogram and fDM analyses were performed on co-registered baseline and post-chemotherapy maps. DTI changes were compared with modifications of tumour area and volume [according to Response Assessment in Neuro-Oncology (RANO) criteria], and seizure response.
Results
After three cycles of TMZ, 20/21 patients were stable according to RANO criteria, but DTI changes were observed in all patients (Wilcoxon test, P ≤ 0.03). After six cycles, DTI changes were more pronounced (P ≤ 0.005). Seventy-five percent of patients had early seizure response with significant improvement of DTI values, maintaining stability on FLAIR. Early changes of the 25th percentiles of p and MD predicted final volume change (R2 = 0.614 and 0.561, P < 0.0005, respectively). TMZ-related changes were located mainly at tumour borders on p and MD fDMs.
Conclusions
DTI-based histogram and fDM analyses are useful techniques to evaluate the early effects of TMZ chemotherapy in LGG patients.
Key Points
• DTI helps to assess the efficacy of chemotherapy in low-grade gliomas.
• Histogram analysis of DTI metrics quantifies structural changes in tumour tissue.
• Functional diffusion maps (fDMs) spatially localize the changes of DTI metrics.
• Changes in DTI histograms and fDMs precede changes in conventional MRI.
• Early changes in DTI histograms and fDMs correlate with seizure response.



Similar content being viewed by others
Abbreviations
- DTI:
-
diffusion tensor imaging
- MRI:
-
magnetic resonance imaging
- fDM:
-
functional diffusion maps
- LGGs:
-
low-grade gliomas
- HGGs:
-
high-grade gliomas
- WM:
-
white matter
- RANO:
-
Response Assessment in Neuro-Oncology
- FLAIR:
-
fluid-attenuated inversion recovery
- TMZ:
-
Temozolomide
- PCV:
-
Procarbazine, Lomustine, and Vincristine
- PR:
-
partial response
- mR:
-
minor response
- SD:
-
stable disease
- PD:
-
progressive disease
- vPR:
-
volumetric partial response
- vmR:
-
volumetric minor response
- vSD:
-
volumetric stable disease
- vPD:
-
volumetric progression of disease
- ADC:
-
apparent diffusion coefficient
- FA:
-
fractional anisotropy
- MD:
-
mean diffusivity
- p :
-
pure isotropic diffusion
- q :
-
pure anisotropic diffusion
- IQR:
-
interquartile range
- ROC:
-
receiver operating characteristic
- AUC:
-
area under the ROC curve
References
Rudà R, Trevisan E, Soffietti R (2012) Low-grade gliomas. Handb Clin Neurol 105:437–450
Castellano A, Bello L, Michelozzi C et al (2012) Role of diffusion tensor magnetic resonance tractography in predicting the extent of resection in glioma surgery. Neuro Oncol 14:192–202
Talos IF, Zou KH, Ohno-Machado L et al (2006) Supratentorial low-grade glioma resectability: Statistical predictive analysis based on anatomic MR features and tumor characteristics. Radiology 239:506–513
Smith JS, Chang EF, Lamborn KR et al (2008) Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol 26:1338–1345
Soffietti R, Baumert BG, Bello L et al (2010) Guidelines on management of low-grade gliomas: Report of an EFNS-EANO task force. Eur J Neurol 17:1124–1133
Keles GE, Lamborn KR, Berger MS (2001) Low-grade hemispheric gliomas in adults: A critical review of extent of resection as a factor influencing outcome. J Neurosurg 95:735–745
Brada M, Viviers L, Abson C et al (2003) Phase II study of primary temozolomide chemotherapy in patients with WHO grade II gliomas. Ann Oncol 14:1715–1721
Pace A, Vidiri A, Galie E et al (2003) Temozolomide chemotherapy for progressive low-grade glioma: Clinical benefits and radiological response. Ann Oncol 14:1722–1726
van den Bent MJ, Afra D, de Witte O et al (2005) Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: The EORTC 22845 randomised trial. Lancet 366:985–990
Duffau H, Taillandier L, Capelle L (2006) Radical surgery after chemotherapy: A new therapeutic strategy to envision in grade II glioma. J Neurooncol 80:171–176
Blonski M, Taillandier L, Herbet G et al (2012) Combination of neoadjuvant chemotherapy followed by surgical resection as a new strategy for WHO grade II gliomas: A study of cognitive status and quality of life. J Neurooncol 106:353–366
Blonski M, Pallud J, Goze C et al (2013) Neoadjuvant chemotherapy may optimize the extent of resection of world health organization grade II gliomas: A case series of 17 patients. J Neurooncol 113:267–275
van den Bent MJ, Wefel JS, Schiff D et al (2011) Response assessment in neuro-oncology (a report of the RANO group): Assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol 12:583–593
Galanis E, Buckner JC, Maurer MJ et al (2006) Validation of neuroradiologic response assessment in gliomas: Measurement by RECIST, two-dimensional, computer-assisted tumor area, and computer-assisted tumor volume methods. Neuro Oncol 8:156–165
Sorensen AG, Patel S, Harmath C et al (2001) Comparison of diameter and perimeter methods for tumor volume calculation. J Clin Oncol 19:551–557
Dempsey MF, Condon BR, Hadley DM (2005) Measurement of tumor "size" in recurrent malignant glioma: 1D, 2D, or 3D? AJNR Am J Neuroradiol 26:770–776
Rudà R, Bello L, Duffau H, Soffietti R (2012) Seizures in low-grade gliomas: Natural history, pathogenesis, and outcome after treatments. Neuro Oncol 14:iv55–64
Price SJ, Pena A, Burnet NG et al (2004) Tissue signature characterisation of diffusion tensor abnormalities in cerebral gliomas. Eur Radiol 14:1909–1917
Goebell E, Paustenbach S, Vaeterlein O et al (2006) Low-grade and anaplastic gliomas: Differences in architecture evaluated with diffusion-tensor MR imaging. Radiology 239:217–222
Stadlbauer A, Nimsky C, Buslei R et al (2007) Diffusion tensor imaging and optimized fiber tracking in glioma patients: Histopathologic evaluation of tumor-invaded white matter structures. Neuroimage 34:949–956
Sternberg EJ, Lipton ML, Burns J (2014) Utility of diffusion tensor imaging in evaluation of the peritumoral region in patients with primary and metastatic brain tumors. AJNR Am J Neuroradiol 35:439–444
Pena A, Green HA, Carpenter TA, Price SJ, Pickard JD, Gillard JH (2006) Enhanced visualization and quantification of magnetic resonance diffusion tensor imaging using the p:Q tensor decomposition. Br J Radiol 79:101–109
Price SJ, Jena R, Burnet NG, Carpenter TA, Pickard JD, Gillard JH (2007) Predicting patterns of glioma recurrence using diffusion tensor imaging. Eur Radiol 17:1675–1684
Price SJ, Jena R, Burnet NG et al (2006) Improved delineation of glioma margins and regions of infiltration with the use of diffusion tensor imaging: An image-guided biopsy study. AJNR Am J Neuroradiol 27:1969–1974
Nowosielski M, Recheis W, Goebel G et al (2011) ADC histograms predict response to anti-angiogenic therapy in patients with recurrent high-grade glioma. Neuroradiology 53:291–302
Pope WB, Qiao XJ, Kim HJ et al (2012) Apparent diffusion coefficient histogram analysis stratifies progression-free and overall survival in patients with recurrent GBM treated with bevacizumab: A multi-center study. J Neurooncol 108:491–498
Hamstra DA, Chenevert TL, Moffat BA et al (2005) Evaluation of the functional diffusion map as an early biomarker of time-to-progression and overall survival in high-grade glioma. Proc Natl Acad Sci U S A 102:16759–16764
Ellingson BM, Malkin MG, Rand SD et al (2010) Validation of functional diffusion maps (fDMs) as a biomarker for human glioma cellularity. J Magn Reson Imaging 31:538–548
Belhawi SM, Hoefnagels FW, Baaijen JC et al (2011) Early postoperative MRI overestimates residual tumour after resection of gliomas with no or minimal enhancement. Eur Radiol 21:1526–1534
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. european organization for research and treatment of cancer, national cancer institute of the united states, national cancer institute of canada. J Natl Cancer Inst 92:205–216
De Nunzio G, Pastore G, Donativi M, Castellano A, Falini A (2011) A CAD system for cerebral glioma based on texture features in DT-MR images. Nucl Instr Meth A. doi:10.1016/j.nima.2010.12.086
Moffat BA, Chenevert TL, Lawrence TS et al (2005) Functional diffusion map: A noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proc Natl Acad Sci U S A 102:5524–5529
O'Connor JP, Jackson A, Asselin MC, Buckley DL, Parker GJ, Jayson GC (2008) Quantitative imaging biomarkers in the clinical development of targeted therapeutics: Current and future perspectives. Lancet Oncol 9:766–776
Sherman JH, Moldovan K, Yeoh HK et al (2011) Impact of temozolomide chemotherapy on seizure frequency in patients with low-grade gliomas. J Neurosurg 114:1617–1621
Jones TL, Byrnes TJ, Yang G, Howe FA, Bell BA, Barrick TR (2014) Brain tumor classification using the diffusion tensor image segmentation (D-SEG) technique. Neuro Oncol. doi:nou 159 [pii]
Khayal IS, McKnight TR, McGue C et al (2009) Apparent diffusion coefficient and fractional anisotropy of newly diagnosed grade II gliomas. NMR Biomed 22:449–455
Hoefnagels FW, De Witt HP, Sanz-Arigita E et al (2014) Differentiation of edema and glioma infiltration: Proposal of a DTI-based probability map. J Neurooncol 120:187–198
Pavlisa G, Rados M, Pavlisa G, Pavic L, Potocki K, Mayer D (2009) The differences of water diffusion between brain tissue infiltrated by tumor and peritumoral vasogenic edema. Clin Imaging 33:96–101
Min ZG, Niu C, Rana N, Ji HM, Zhang M (2013) Differentiation of pure vasogenic edema and tumor-infiltrated edema in patients with peritumoral edema by analyzing the relationship of axial and radial diffusivities on 3.0T MRI. Clin Neurol Neurosurg 115:1366–1370
Zamecnik J (2005) The extracellular space and matrix of gliomas. Acta Neuropathol 110:435–442
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Acknowledgments
The scientific guarantor of this publication is Andrea Falini. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. This study has received funding by the Italian Ministry of Health (RF-2009-1530888) and by the Italian Ministry of Education, University, and Research (MIUR PON 254/Ric “Ricerca e competitività 2007-2013”, upgrading of the “Centro ricerche per la salute dell'uomo e dell'ambiente” PONa3_00334). M.R. has received funding by the Fellowship for Abroad 2013 of the Fondazione Italiana per la Ricerca sul Cancro (FIRC). Antonella Castellano conducted this study as partial fulfillment of her PhD in Molecular Medicine, Program in Experimental Neurology, Vita-Salute San Raffaele University, Milan, Italy.
The authors are grateful to Prof. Alessandro Ambrosi that kindly provided statistical advice for this manuscript and Kesshi M. Jordan for English editing. Institutional review board approval was obtained.
Written informed consent was obtained from all patients in this study. Methodology: retrospective, diagnostic study, observational.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 6.52 mb)
Rights and permissions
About this article
Cite this article
Castellano, A., Donativi, M., Rudà, R. et al. Evaluation of low-grade glioma structural changes after chemotherapy using DTI-based histogram analysis and functional diffusion maps. Eur Radiol 26, 1263–1273 (2016). https://doi.org/10.1007/s00330-015-3934-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3934-6