Abstract
Objectives
To evaluate lung T2 mapping for quantitative characterization and differentiation of ground-glass opacity (GGO), reticulation (RE) and honeycombing (HC) in usual interstitial pneumonia (UIP) and non-specific interstitial pneumonia (NSIP).
Methods
Twelve patients with stable UIP or NSIP underwent thin-section multislice CT and 1.5-T MRI of the lung. A total of 188 regions were classified at CT into normal (n = 29) and pathological areas, including GGO (n = 48), RE (n = 60) and HC (n = 51) predominant lesions. Entire lung T2 maps based on multi-echo single shot TSE sequence (TE: 20, 40, 79, 140, 179 ms) were generated from each subject with breath-holds at end-expiration and ECG-triggering.
Results
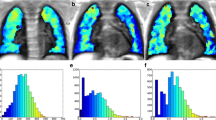
The median T2 relaxation of GGO was 67 ms (range 60-72 ms). RE predominant lesions had a median relaxation of 74 ms (range 69-79 ms), while for HC pattern this was 79 ms (range 74-89 ms). The median T2 relaxation for normal lung areas was 41 ms (ranged 38-49 ms), and showed significant difference to pathological areas (p < 0.001). A statistical difference was found between the T2 relaxation of GGO, RE and HC (p < 0.05).
Conclusions
The proposed method provides quantitative information for pattern differentiation, potentially allowing for monitoring of progression and response to treatment, in interstitial lung disease.
Key points
• Multi-echo single shot TSE sequence allows for entire lung T2 mapping.
• Lung remodelling patterns in ILD show different T2 relaxation.
• Quantitative T2 mapping may provide information for monitoring of ILD.


Similar content being viewed by others
Abbreviations
- GGO:
-
Ground-glass opacity
- HC:
-
Honeycombing
- ILD:
-
Interstitial lung disease
- NSIP:
-
Non-specific interstitial pneumonia
- RE:
-
Reticulation
- ROI:
-
Region-of-interest
- UIP:
-
Usual interstitial pneumonia
References
Wallace WA, Fitch PM, Simpson AJ, Howie SE (2007) Inflammation-associated remodelling and fibrosis in the lung - a process and an end point. Int J Exp Pathol 88:103–110
Raghu G, Collard HR, Egan JJ, on behalf of the ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis et al (2011) An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183:788–824
Prosch H, Schaefer-Prokop CM, Eisenhuber E, Kienzl D, Herold CJ (2013) CT protocols ininterstitial lung diseases–a survey among members of the European Society of ThoracicImaging and a review of the literature. Eur Radiol 23:1553–1563
Ohno Y, Takenaka D, Kanda T et al (2012) Adaptive iterative dose reduction using 3D processing for reduced- and low-dose pulmonary CT: comparison with standard-dose CT for image noise reduction and radiological findings. AJR 199:W477–W485
Christe A, Charimo-Torrente J, Roychoudhury K, Vock P, Roos JE (2013) Accuracy of low-dose computed tomography (CT) for detecting and characterizing the most common CT-patterns of pulmonary disease. Eur J Radiol 82:e142–e150
Yokoyama A, Kondo K, Nakajima M et al (2006) Prognostic value of circulating KL-6 in idiopathic pulmonary fibrosis. Respirology 11:164–168
Richards TJ, Kaminski N, Baribaud F et al (2012) Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 185:67–76
Walsh SL, Hansell DM (2010) Diffuse interstitial lung disease: overlaps and uncertainties. Eur Radiol 20:1859–1867
Schaefer-Prokop C, Prokop M, Fleischmann D, Herold C (2001) High-resolution CT of diffuse interstitial lung disease: key findings in common disorders. Eur Radiol 11:373–392
Engeler CE, Tashjian JH, Trenkner SW, Walsh JW (1993) Ground-glass opacity of the lung parenchyma: a guide to analysis with high-resolution CT. AJR Am J Roentgenol 160:249–251
Gruden JF, Panse PM, Leslie KO, Tazelaar HD, Colby TV (2013) UIP diagnosed at surgical lung biopsy, 2000-2009: HRCT patterns and proposed classification system. AJR Am J Roentgenol 200:W458–W467
Jakob PM, Hillenbrand CM, Wang T, Schultz G, Hahn D, Haase A (2001) Rapid quantitative lung (1)H T(1) mapping. J Magn Reson Imaging 14:795–799
Cutillo AG, Chan PH, Ailion DC et al (2002) Characterization of bleomycin lung injury by nuclear magnetic resonance: correlation between NMR relaxation times and lung water and collagen content. Magn Reson Med 47:246–256
Bauman G, Puderbach M, Deimling M et al (2009) Non-contrast-enhanced perfusion and ventilation assessment of the human lung by means of fourier decomposition in proton MRI. Magn Reson Med 62:656–664
Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J (2008) Fleischner society: glossary of terms for thoracic imaging. Radiology 246:697–722
Skalina S, Kundel HL, Wolf G, Marshall B (1984) The effect of pulmonary edema on proton nuclear magnetic resonance relaxation times. Investig Radiol 19:7–9
Newcomb CH, Van Dyk J, Hill RP (1994) The role of magnetic resonance for assessing radiation-induced lung damage. Int J Radiat Oncol Biol Phys 30:125–132
Jacob RE, Amidan BG, Soelberg J, Minard KR (2010) In vivo MRI of altered proton signal intensity and T2 relaxation in a bleomycin model of pulmonary inflammation and fibrosis. J Magn Reson Imaging 31:1091–1099
Estilaei M, MacKay A, Whittall K, Mayo J (1999) In vitro measurements of water content and T2 relaxation times in lung using a clinical MRI scanner. J Magn Reson Imaging 9:699–703
Huber DJ, Kobzik L, Melanson G, Adams DF (1985) The detection of inflammation in collapsed lung by alterations in proton nuclear magnetic relaxation times. Investig Radiol 20:460–464
Mayo JR, MacKay AL, Whittall KP, Baile EM, Paré PD (1995) Measurement of lung water content and pleural pressure gradient with magnetic resonance imaging. J Thorac Imaging 10:73–81
Cannie M, Jani J, De Keyzer F, Roebben I, Breysem L, Deprest J (2011) T2 quantifications of fetal lungs at MRI-normal ranges. Prenat Diagn 31:705–711
Adams EW, Counsell SJ, Hajnal JV et al (2002) Magnetic resonance imaging of lung water content and distribution in term and preterm infants. Am J Respir Crit Care Med 166:397–402
Hatabu H, Alsop DC, Listerud J, Bonnet M, Gefter WB (1999) T2* and proton density measurement of normal human lung parenchyma using submillisecond echo time gradient echo magnetic resonance imaging. Eur J Radiol 29:245–252
Stock KW, Chen Q, Hatabu H, Edelman RR (1999) Magnetic resonance T2* measurements of the normal human lung in vivo with ultra-short echo times. Magn Reson Imaging 17:997–1000
Ohno Y, Nishio M, Koyama H et al (2013) Pulmonary MR imaging with ultra-short TEs: utility for disease severity assessment of connective tissue disease patients. Eur J Radiol 82:1359–1365
Stadler A, Jakob PM, Griswold M, Barth M, Bankier AA (2005) T1 mapping of the entire lung parenchyma: influence of the respiratory phase in healthy individuals. J Magn Reson Imaging 21:759–764
Stadler A, Jakob PM, Griswold M, Stiebellehner L, Barth M, Bankier AA (2008) T1 mapping of the entire lung parenchyma: influence of respiratory phase and correlation to lung function test results in patients with diffuse lung disease. Magn Reson Med 59:96–101
Caravan P, Yang Y, Zachariah R et al (2013) Molecular magnetic resonance imaging of pulmonary fibrosis in mice. Am J Respir Cell Mol Biol 49:1120–1126
Ohno Y, Koyama H, Yoshikawa T (2011) Pulmonary magnetic resonance imaging for airway diseases. J Thorac Imaging 26:301–316
Lutterbey G, Gieseke J, von Falkenhausen M, Morakkabati N, Schild H (2005) Lung MRI at 3.0 T: a comparison of helical CT and high-field MRI in the detection of diffuse lung disease. Eur Radiol 15:324–328
Sung A, Swigris J, Saleh A, Raoof S (2007) High-resolution chest tomography in idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia: utility and challenges. Curr Opin Pulm Med 13:451–457
McFadden RG, Carr TJ, Wood TE (1987) Proton magnetic resonance imaging to stage activity of interstitial lung disease. Chest 92:31–39
Felício CH, Parra ER, Capelozzi VL (2007) Idiopathic and collagen vascular disease nonspecific interstitial pneumonia: clinical significance of remodeling process. Lung 185:39–46
Mulkern R, Haker S, Mamata H et al (2014) Lung parenchymal signal intensity in MRI: a technical review with educational aspirations regarding reversible versus irreversible transverse relaxation effects in common pulse sequences. Concepts Magn Reson Part A Bridg Educ Res 43A:29–53
Hatabu H, Gaa J, Tadamura E et al (1999) MR imaging of pulmonary parenchyma with a half-Fourier single-shot turbo spin-echo (HASTE) sequence. Eur J Radiol 29:152–159
Ellis SM, Hansell DM (2002) Idiopathic interstitial pneumonias: imaging-pathology correlation. Eur Radiol 12:610–626
Sumikawa H, Johkoh T, Ichikado K et al (2009) Nonspecific interstitial pneumonia: histologic correlation with high-resolution CT in 29 patients. Eur J Radiol 70:35–40
Martinez FJ, Flaherty K (2006) Pulmonary function testing in idiopathic interstitial pneumonias. Proc Am Thorac Soc 3:315–321
Acknowledgments
The authors would like to thank the ERASMUS exchange program for PhD students. The scientific guarantor of this publication is Julien Dinkel. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Methodology: prospective, experimental study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buzan, M.T.A., Eichinger, M., Kreuter, M. et al. T2 mapping of CT remodelling patterns in interstitial lung disease. Eur Radiol 25, 3167–3174 (2015). https://doi.org/10.1007/s00330-015-3751-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3751-y