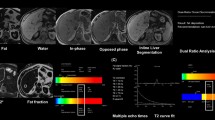
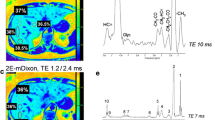
Abstract
Objectives
To evaluate the automated two-point Dixon screening sequence for the detection and estimated quantification of hepatic iron and fat compared with standard sequences as a reference.
Methods
One hundred and two patients with suspected diffuse liver disease were included in this prospective study. The following MRI protocol was used: 3D-T1-weighted opposed- and in-phase gradient echo with two-point Dixon reconstruction and dual-ratio signal discrimination algorithm (“screening” sequence); fat-saturated, multi-gradient-echo sequence with 12 echoes; gradient-echo T1 FLASH opposed- and in-phase. Bland–Altman plots were generated and correlation coefficients were calculated to compare the sequences.
Results
The screening sequence diagnosed fat in 33, iron in 35 and a combination of both in 4 patients. Correlation between R2* values of the screening sequence and the standard relaxometry was excellent (r = 0.988). A slightly lower correlation (r = 0.978) was found between the fat fraction of the screening sequence and the standard sequence. Bland–Altman revealed systematically lower R2* values obtained from the screening sequence and higher fat fraction values obtained with the standard sequence with a rather high variability in agreement.
Conclusions
The screening sequence is a promising method with fast diagnosis of the predominant liver disease. It is capable of estimating the amount of hepatic fat and iron comparable to standard methods.
Key points
• MRI plays a major role in the clarification of diffuse liver disease.
• The screening sequence was introduced for the assessment of diffuse liver disease.
• It is a fast and automated algorithm for the evaluation of hepatic iron and fat.
• It is capable of estimating the amount of hepatic fat and iron.







Similar content being viewed by others
Abbreviations
- FF:
-
fat fraction
- HIO:
-
hepatic iron overload
- NAFLD:
-
non-alcoholic fatty liver disease
- MRI:
-
magnetic resonance imaging
References
Harrison SA, Neuschwander-Tetri BA (2004) Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Clin Liver Dis 8:861–879, ix
Clark JM, Diehl AM (2003) Defining nonalcoholic fatty liver disease: implications for epidemiologic studies. Gastroenterology 124:248–250
Moirand R, Mortaji AM, Loreal O, Paillard F, Brissot P, Deugnier Y (1997) A new syndrome of liver iron overload with normal transferrin saturation. Lancet 349:95–97
Bonkovsky HL, Jawaid Q, Tortorelli K et al (1999) Non-alcoholic steatohepatitis and iron: increased prevalence of mutations of the HFE gene in non-alcoholic steatohepatitis. J Hepatol 31:421–429
Henninger B, Kremser C, Rauch S et al (2013) Evaluation of liver fat in the presence of iron with MRI using T2* correction: a clinical approach. Eur Radiol 23:1643–1649
Kew MC (2009) Hepatic iron overload and hepatocellular carcinoma. Cancer Lett 286:38–43
Hernando D, Levin YS, Sirlin CB, Reeder SB (2014) Quantification of liver iron with MRI: state of the art and remaining challenges. J Magn Reson Imaging 40:1003–1021
Reeder SB, Sirlin CB (2010) Quantification of liver fat with magnetic resonance imaging. Magn Reson Imaging Clin N Am 18:337–357, ix
Bashir MR, Zhong X, Dale BM, Gupta RT, Boll DT, Merkle EM (2013) Automated patient-tailored screening of the liver for diffuse steatosis and iron overload using MRI. AJR Am J Roentgenol 201:583–588
Bashir MR, Merkle EM, Smith AD, Boll DT (2012) Hepatic MR imaging for in vivo differentiation of steatosis, iron deposition and combined storage disorder: single-ratio in/opposed phase analysis vs. dual-ratio Dixon discrimination. Eur J Radiol 81:e101–e109
Boll DT, Marin D, Redmon GM, Zink SI, Merkle EM (2010) Pilot study assessing differentiation of steatosis hepatis, hepatic iron overload, and combined disease using two-point dixon MRI at 3 T: in vitro and in vivo results of a 2D decomposition technique. AJR Am J Roentgenol 194:964–971
Bashir MR, Dale BM, Merkle EM, Boll DT (2012) Automated liver sampling using a gradient dual-echo Dixon-based technique. Magn Reson Med 67:1469–1477
Breuer FA, Blaimer M, Heidemann RM, Mueller MF, Griswold MA, Jakob PM (2005) Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging. Magn Reson Med 53:684–691
Jellus V (2010) Phase correction method. US20100201364 A1
Liu CY, McKenzie CA, Yu H, Brittain JH, Reeder SB (2007) Fat quantification with IDEAL gradient echo imaging: correction of bias from T(1) and noise. Magn Reson Med 58:354–364
Yang IY, Cui Y, Wiens CN, Wade TP, Friesen-Waldner LJ, McKenzie CA (2014) Fat fraction bias correction using T1 estimates and flip angle mapping. J Magn Reson Imaging 39:217–223
Szczepaniak LS, Babcock EE, Schick F et al (1999) Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol 276:E977–E989
Hankins JS, McCarville MB, Loeffler RB et al (2009) R2* magnetic resonance imaging of the liver in patients with iron overload. Blood 113:4853–4855
St Pierre TG, Clark PR, Chua-anusorn W et al (2005) Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood 105:855–861
Henninger B, Kremser C, Rauch S et al (2012) Evaluation of MR imaging with T1 and T2* mapping for the determination of hepatic iron overload. Eur Radiol 22:2478–2486
Cicchetti DV, Feinstein AR (1990) High agreement but low kappa: II. Resolving the paradoxes. J Clin Epidemiol 43:551–558
Watkins MW, Pacheco M (2000) Interobserver agreement in behavioral research: importance and calculation. J Behav Educ 10:205–2012
Robin X, Turck N, Hainard A et al (2011) pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinforma 12:77
Meisamy S, Hines CD, Hamilton G et al (2011) Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology 258:767–775
Yokoo T, Shiehmorteza M, Hamilton G et al (2011) Estimation of hepatic proton-density fat fraction by using MR imaging at 3.0 T. Radiology 258:749–759
Bydder M, Yokoo T, Hamilton G et al (2008) Relaxation effects in the quantification of fat using gradient echo imaging. Magn Reson Imaging 26:347–359
Kuhn JP, Evert M, Friedrich N et al (2011) Noninvasive quantification of hepatic fat content using three-echo Dixon magnetic resonance imaging with correction for T2* relaxation effects. Invest Radiol 46:783–789
Deugnier Y, Turlin B (2007) Pathology of hepatic iron overload. World J Gastroenterol 13:4755–4760
Wood JC, Enriquez C, Ghugre N et al (2005) MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood 106:1460–1465
Yu H, Shimakawa A, Hines CD et al (2011) Combination of complex-based and magnitude-based multiecho water-fat separation for accurate quantification of fat-fraction. Magn Reson Med 66:199–206
Hines CD, Yu H, Shimakawa A, McKenzie CA, Brittain JH, Reeder SB (2009) T1 independent, T2* corrected MRI with accurate spectral modeling for quantification of fat: validation in a fat-water-SPIO phantom. J Magn Reson Imaging 30:1215–1222
Kuhn JP, Hernando D, Munoz del Rio A et al (2012) Effect of multipeak spectral modeling of fat for liver iron and fat quantification: correlation of biopsy with MR imaging results. Radiology 265:133–142
Zhong X, Nickel MD, Kannengiesser SA, Dale BM, Kiefer B, Bashir MR (2013) Liver fat quantification using a multi-step adaptive fitting approach with multi-echo GRE imaging. Magn Reson Med 72:1353–1365
McCarville MB, Hillenbrand CM, Loeffler RB et al (2010) Comparison of whole liver and small region-of-interest measurements of MRI liver R2* in children with iron overload. Pediatr Radiol 40:1360–1367
Positano V, Salani B, Pepe A et al (2009) Improved T2* assessment in liver iron overload by magnetic resonance imaging. Magn Reson Imaging 27:188–197
Acknowledgments
The scientific guarantor of this publication is Dr. Benjamin Henninger. The authors of this manuscript declare relationships with the following companies: S. Kannengiesser, X. Zhong and G. Reiter are employees of Siemens Healthcare. They had no control of all data for the duration of the study. All the other authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. One of the authors has statistical experience (C. Kremser). Nevertheless no complex statistical methods were necessary for this paper. Institutional review board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Methodology: prospective, diagnostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Henninger, B., Zoller, H., Rauch, S. et al. Automated two-point dixon screening for the evaluation of hepatic steatosis and siderosis: comparison with R2*-relaxometry and chemical shift-based sequences. Eur Radiol 25, 1356–1365 (2015). https://doi.org/10.1007/s00330-014-3528-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3528-8