Abstract
Objectives
To evaluate the quantitative parameters obtained from dynamic MR T2*-weighted images as predictors of survival taking into consideration the biasing effects of other survival-related covariates.
Methods
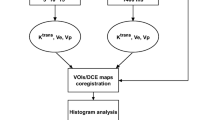
Thirty-nine patients (60 ± 14 years; survival 267 ± 191 days) with high-grade gliomas (8 grade III, 31 grade IV) were retrospectively included in the study. Additional data incorporated Karnofsky performance scale, tumour resection extension after surgery and type of treatment. Dynamic T2*-weighted MRI was acquired before treatment. Tumour curves were extracted for each voxel, and several quantitative parameters were obtained from the whole tumour volume and the 10 % maximum values. Additional image covariates included the presence of necrosis, single or multiple lesions, and tumour and oedema volumes. The relationship between quantitative parameters and survival was assessed using clusterisation techniques and the log-rank method. Cox regression analysis was used to evaluate each parameter’s predictive value.
Results
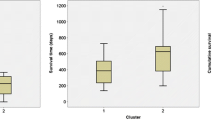
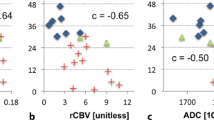
Only the mean of the 10 % maximum values of the transfer coefficient showed an independent relationship with patient survival (log-rank chi-squared test <0.001, Cox regression P = 0.015), with higher values corresponding to lower survival rates.
Conclusions
High maximum transfer coefficient values show an independent statistical relationship with low survival in high-grade glioma patients. This imaging biomarker can be used as a predictor of prognosis.
Key Points
• Histological examination is the standard procedure for predicting glioma biological behaviour.
• Tumour biopsies may be biased by sample size and location.
• Dynamic T2*-weighted MRI quantitative analysis characterises tumour vasculature at the voxel level.
• High-transfer constant maximum values are independent predictors of low overall survival.





Similar content being viewed by others
References
Folkerth RD (2004) Histologic measures of angiogenesis in human primary brain tumors. Cancer Treat Res 117:79–95
Burger P (1986) Malignant astrocytic neoplasms: classification, pathology, anatomy, and response to therapy. Semin Oncol 13:16–20
Daumas-Duport C, Scheithauer B, O’Fallon J, Kelly P (1988) Grading of astrocytomas: a simple and reproducible method. Cancer 62:2152–2165
Kleihues P, Burger PC, Scheithauer BW (1993) The new WHO classification of brain tumors. Brain Pathol 3:255–268
Kleihues P, Cavenee WK (2000) World Health Organization classification of tumors: Pathology and genetics of tumors of the nervous system. IARC Press, Lyon
Brat DJ, Parisi JE, Kleinschmidt-DeMasters BK et al (2008) Surgical neuropathology update: a review of changes introduced by the WHO classification of tumours of the central nervous system, 4th edition. Arch Pathol Lab Med 132:993–1007
Kepes JJ (1994) Pitfalls and problems in the histopathologic evaluation of stereotactic needle biopsy specimens. Neurosurg Clin N Am 5:19–33
Sawaya R, Hammoud M, Schoppa D et al (1998) Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery 42:1044–1055
Waldman AD, Jackson A, Price SJ et al (2009) Quantitative imaging biomarkers in neuro-oncology. Nat Rev Clin Oncol 6:445–454
Law M, Yang S, Wang H et al (2003) Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol 24:1989–1998
Ostergaard L, Weisskoff RM, Chesler DA et al (1996) High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: mathematical approach and statistical analysis. Magn Reson Med 36:715–725
Ostergaard L, Sorensen AG, Kwong KK et al (1996) High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part II: experimental comparison and preliminary results. Magn Reson Med 36:726–736
Aronen HJ, Gazit IE, Louis DN et al (1994) Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology 191:41–51
Knopp EA, Cha S, Johnson G et al (1999) Glial neoplasms: dynamic contrast-enhanced T2*-weighted MR imaging. Radiology 211:791–798
Sourbron SP, Buckley DL (2012) Tracer kinetic modeling in MRI: estimating perfusion and capillary permeability. Phys Med Biol 57:1–33
Li KL, Zhu XP, Waterton J et al (2000) Improved 3D quantitative mapping of blood volume and endothelial permeability in brain tumors. J Magn Reson Imaging 12:347–357
Law M, Yang S, Babb et al (2004) Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol 25:746–755
Law M, Young R, Babb J et al (2006) Comparing perfusion metrics obtained from a single compartment versus pharmacokinetic modeling methods using dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol 27:1975–1978
Johnson G, Wetzel SG, Cha S, Babb J, Tofts PS (2004) Measuring blood volume and vascular transfer constant from dynamic, T2*-weighted contrast-enhanced MRI. J Magn Reson Imaging 51:961–968
Mills SJ, Patankar TA, Haroon HA et al (2006) Do cerebral blood volume and contrast transfer coefficient predict prognosis in human glioma? AJNR Am J Neuroradiol 27:853–858
Law M, Young RJ, Babb JS et al (2008) Gliomas. Predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 247:490–498
Hirai T, Murakami R, Nakamura H et al (2008) Prognostic value of perfusion MR imaging of high-grade astrocytomas: long-term follow-up study. AJNR Am J Neuroradiol 29:1505–1510
Albert FK, Forsting M, Sartor K, Adams HP, Kunze S (1994) Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery 34:45–60
Emblem KE, Nedregaard B, Nome T et al (2008) Glioma grading by using histogram analysis of blood volume heterogeneity from MR-derived cerebral blood volume maps. Radiology 247:808–817
Lev M, Ozsunar Y, Henson J et al (2004) Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendrogliomas. AJNR Am J Neuroradiol 25:214–221
Calamante F, Vonken EPA, Van Osch MJP (2007) Contrast agent concentration measurements affecting quantification of bolus tracking perfusion MRI. Magn Reson Med 58:544–553
Wong JC, Provenzale JM, Petrella JR (2000) Perfusion MR imaging of brain neoplasms. AJR Am J Roentgenol 174:1147–1157
Revert Ventura AJ, Sanz-Requena R, Martí-Bonmatí L et al (2010) Nosological analysis of MRI tissue perfusion parameters obtained using the unicompartmental and pharmacokinetic models in cerebral glioblastomas. Radiologia 52:432–441
Zhang T, Ramakrishnon R, Livny (1996) BIRCH: an efficient data clustering method for very large databases. Proceedings of the ACM SIGMOD Conference on Management of Data, Montreal, Canada, pp 103-104
Scott CB, Scarantino C, Urtasun R et al (1998) Validation and predictive power of Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis classes for malignant glioma patients: a report using RTOG90–06. Int J Radiat Oncol Biol Phys 40:51–55
Bauman G, Lote K, Larson D et al (1999) Pretreatment factors predict overall survival for patients with low-grade glioma: a recursive partitioning analysis. Int J Radiat Oncol Biol Phys 45:923–929
Cha S (2006) Update on brain tumor imaging: from anatomy to physiology. AJNR Am J Neuroradiol 27:475–487
Pierallini A, Bonamini M, Pantano P et al (1998) Radiological assessment of necrosis in glioblastoma: variability and prognostic value. Neuroradiology 40:150–153
Sugahara T, Korogi Y, Kochi M et al (1999) Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging 9:53–60
Shin JH, Lee HK, Kwun BD et al (2002) Using relative cerebral blood flow and volume to evaluate the histopathologic grade of cerebral gliomas: preliminary results. AJR Am J Roentgenol 179:783–789
Bulakbasi N, Kocaoglu M, Farzaliyev A et al (2005) Assessment of diagnostic accuracy of perfusion MR imaging in primary and metastatic solitary malignant brain tumors. AJNR Am J Neuroradiol 26:2187–2199
Roberts HC, Roberts TPL, Brasch RC et al (2000) Quantitative measurement of microvascular permeability in human brain tumors achieved using dynamic contrast-enhanced MR imaging: correlation with histologic grade. AJNR Am J Neuroradiol 21:891–899
Ludemann L, Gieger W, Wurm R et al (2001) Comparison of dynamic contrast-enhanced MRI with WHO tumor grading for gliomas. Eur Radiol 11:1231–1241
Provenzale JM, Wang GR, Brenner T et al (2002) Comparison of permeability in high-grade and low-grade brain tumors using dynamic susceptibility contrast MR Imaging. AJR Am J Roentgenol 178:711–716
Patankar TF, Haroon HA, Mills SJ et al (2005) Is volume transfer coefficient K(trans) related to histologic grade in human gliomas? AJNR Am J Neuroradiol 26:2455–2465
Leon SP, Folkerth RD, Black PM (1996) Microvessel density is a prognostic indicator for patients with astroglial brain tumors. Cancer 77:362–372
Abdulrauf SI, Edvardsen K, Ho KL et al (1998) Vascular endothelial growth factor expression and vascular density as prognostic markers of survival in patients with low-grade astrocytomas. J Neurosurg 88:513–520
Law M, Young R, Babb J, Pollack E, Johnson G (2007) Histogram analysis versus region of interest analysis of dynamic susceptibility contrast perfusion MR imaging data in the grading of cerebral gliomas. AJNR Am J Neuroradiol 28:761–766
Jackson RJ, Fuller GN, Abi-Said D et al (2001) Limitations of stereotactic biopsy in the initial management of gliomas. Neuro Oncol 3:193–200
Jain R, Narang J, Griffith B et al (2013) Prognostic vascular imaging biomarkers in high-grade gliomas: tumor permeability as an adjunct to blood volume estimates. Acad Radiol 20:478–485
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sanz-Requena, R., Revert-Ventura, A., Martí-Bonmatí, L. et al. Quantitative MR perfusion parameters related to survival time in high-grade gliomas. Eur Radiol 23, 3456–3465 (2013). https://doi.org/10.1007/s00330-013-2967-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-013-2967-y