Abstract
Objectives
To retrospectively identify morphological and physiological post-operative magnetic resonance imaging (MRI) characteristics predictive of glioblastoma recurrences after gross total resection (gross-TR).
Methods
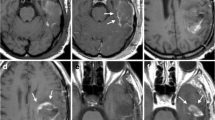
Resection margins of 24 glioblastoma were analysed immediately post-operatively (MRI ≤ 2 h) and early post-operatively (24 h ≤ MRI ≤ 48 h), and subdivided into areas with and without subtle contrast enhancement previously considered non-specific. On follow-up MRI, tumour regrowth areas were subdivided according to recurrence extent (focally/extended) and delay (≤6 and ≥12 months). Co-registration of pre-operative, immediately post-operative and early post-operative MRI with the first follow-up MRI demonstrating recurrence authorised their morphological (contrast enhancements) and physiological (rCBV) characterisation.
Results
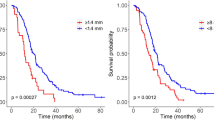
Morphologically, on immediately post-operative MRI, micro-nodular and frayed enhancements correlate significantly with early recurrences (≤6 months). After gross-TR the absence of these enhancements is associated with a significant increase in progression-free survival (61 vs 15 weeks respectively) and overall survival (125 vs 51 weeks respectively). Physiologically, areas with a future focal recurrence have a trend toward higher rCBV than other areas.
Conclusion
Immediately post-operative topography of micro-nodular and frayed enhancements is suggestive of recurrence location and delay. Absence of such enhancements is associated with a fourfold increase in progression-free survival and a 2.5-fold increase in overall survival.
Key Points
• Immediately post-operative MRI reveals contrast enhancement after glioblastoma gross total resection.
• Immediately post-operative micro-nodular and frayed enhancement correlate with early recurrence.
• Absence of micro-nodular/frayed enhancement is associated with 61 weeks’ progression-free survival.
• Absence of micro-nodular/frayed enhancement is associated with 125 weeks’ overall survival.








Similar content being viewed by others
Abbreviations
- Gd:
-
Gadolinium
- (n)TG:
-
(no) tumour growth
- Op:
-
Operative
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- PPV:
-
Positive predictive value
- PWI:
-
Perfusion-weighted imaging
- rCBV:
-
Relative cerebral blood volume
- ROI:
-
Region of interest
- TR:
-
Total resection
References
States Central Brain Tumor Registry of the United (2010) CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2004–2006. Central Brain Tumor Registry of the United States (CBTRUS), Chicago. Available via http://www.cbtrus.org/2010-NPCR-SEER/CBTRUS-WEBREPORT-Final-3-2-10.pdf. Accessed 05 May 2010
Albert FK, Forsting M, Sartor K, Adams HP, Kunze S (1994) Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery 34:45–60, discussion 60-41
Allahdini F, Amirjamshidi A, Reza-Zarei M, Abdollahi M (2010) Evaluating the prognostic factors effective on the outcome of patients with glioblastoma multiformis: does maximal resection of the tumor lengthen the median survival? World Neurosurg 73:128–134, discussion e116
Sanai N, Polley MY, Mcdermott MW, Parsa AT, Berger MS (2011) An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115:3–8
Lacroix M, Abi-Said D, Fourney DR et al (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95:190–198
Hentschel SJ, Sawaya R (2003) Optimizing outcomes with maximal surgical resection of malignant gliomas. Cancer Control 10:109–114
Kuhnt D, Becker A, Ganslandt O, Bauer M, Buchfelder M, Nimsky C (2011) Correlation of the extent of tumor volume resection and patient survival in surgery of glioblastoma multiforme with high-field intraoperative MRI guidance. Neuro Oncol 13:1339–1348
Jeremic B, Grulicic D, Samardzic M et al (1997) The effect of extent of tumor resection on the outcome of combined therapy in patients with glioblastoma multiforme. Srp Arh Celok Lek 125:93–98
Ushio Y, Kochi M, Hamada J, Kai Y, Nakamura H (2005) Effect of surgical removal on survival and quality of life in patients with supratentorial glioblastoma. Neurol Med Chir (Tokyo) 45:454–460, discussion 460-451
Vidiri A, Carapella CM, Pace A et al (2006) Early post-operative MRI: correlation with progression-free survival and overall survival time in malignant gliomas. J Exp Clin Cancer Res 25:177–182
Stewart LA (2002) Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet 359:1011–1018
Li SW, Qiu XG, Chen BS et al (2009) Prognostic factors influencing clinical outcomes of glioblastoma multiforme. Chin Med J (Engl) 122:1245–1249
Mcgirt MJ, Chaichana KL, Gathinji M et al (2009) Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg 110:156–162
Murakami R, Hirai T, Nakamura H et al (2010) Recurrence patterns of glioblastoma treated with postoperative radiation therapy: relationship between extent of resection and progression-free interval. Int J Radiat Oncol Biol Phys 78(3 Suppl 1):S289–S290
Ewelt C, Goeppert M, Rapp M, Steiger HJ, Stummer W, Sabel M (2011) Glioblastoma multiforme of the elderly: the prognostic effect of resection on survival. J Neurooncol 103:611–618
Massey V, Wallner KE (1990) Patterns of second recurrence of malignant astrocytomas. Int J Radiat Oncol Biol Phys 18:395–398
Hess CF, Schaaf JC, Kortmann RD, Schabet M, Bamberg M (1994) Malignant glioma: patterns of failure following individually tailored limited volume irradiation. Radiother Oncol 30:146–149
Wick W, Stupp R, Beule AC et al (2008) A novel tool to analyze MRI recurrence patterns in glioblastoma. Neuro Oncol 10:1019–1024
Schaefer PW, Grant PE, Gonzalez RG (2000) Diffusion-weighted MR imaging of the brain. Radiology 217:331–345
Di Costanzo A, Scarabino T, Trojsi F et al (2006) Multiparametric 3T MR approach to the assessment of cerebral gliomas: tumor extent and malignancy. Neuroradiology 48:622–631
Khan RB, Gutin PH, Rai SN, Zhang L, Krol G, Deangelis LM (2006) Use of diffusion weighted magnetic resonance imaging in predicting early postoperative outcome of new neurological deficits after brain tumor resection. Neurosurgery 59:60–66, discussion 60–66
Pirzkall A, Mcgue C, Saraswathy S et al (2009) Tumor regrowth between surgery and initiation of adjuvant therapy in patients with newly diagnosed glioblastoma. Neuro Oncol 11:842–852
Khayal IS, Polley MY, Jalbert L et al (2010) Evaluation of diffusion parameters as early biomarkers of disease progression in glioblastoma multiforme. Neuro Oncol 12:908–916
Blasel S, Franz K, Ackermann H, Weidauer S, Zanella F, Hattingen E (2011) Stripe-like increase of rCBV beyond the visible border of glioblastomas: site of tumor infiltration growing after neurosurgery. J Neurooncol 103:575–584
Li Y, Lupo JM, Polley MY et al (2011) Serial analysis of imaging parameters in patients with newly diagnosed glioblastoma multiforme. Neuro Oncol 13:546–557
Stecco A, Pisani C, Quarta R et al (2011) DTI and PWI analysis of peri-enhancing tumoral brain tissue in patients treated for glioblastoma. J Neurooncol 102:261–271
Leysalle A, Haberer S (2010) Mise au point sur la pseudoprogression après chimioradiothérapie dans les glioblastomes. Oncologie 12:559–564
Jankovski A, Francotte F, Vaz G et al (2008) Intraoperative magnetic resonance imaging at 3-T using a dual independent operating room-magnetic resonance imaging suite: development, feasibility, safety, and preliminary experience. Neurosurgery 63:412–424, discussion 424–416
Streiner DL, Norman GR (2008) Health measurement scales: a practical guide to their development and use. Oxford University Press, Oxford
Knauth M, Aras N, Wirtz CR, Dorfler A, Engelhorn T, Sartor K (1999) Surgically induced intracranial contrast enhancement: potential source of diagnostic error in intraoperative MR imaging. AJNR Am J Neuroradiol 20:1547–1553
Ekinci G, Akpinar IN, Baltacioglu F et al (2003) Early-postoperative magnetic resonance imaging in glial tumors: prediction of tumor regrowth and recurrence. Eur J Radiol 45:99–107
Keles GE, Anderson B, Berger MS (1999) The effect of extent of resection on time to tumor progression and survival in patients with glioblastoma multiforme of the cerebral hemisphere. Surg Neurol 52:371–379
Aronen HJ, Gazit IE, Louis DN et al (1994) Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology 191:41–51
Rollin N, Guyotat J, Streichenberger N, Honnorat J, Tran Minh VA, Cotton F (2006) Clinical relevance of diffusion and perfusion magnetic resonance imaging in assessing intra-axial brain tumors. Neuroradiology 48:150–159
Sehgal V, Delproposto Z, Haddar D et al (2006) Susceptibility-weighted imaging to visualize blood products and improve tumor contrast in the study of brain masses. J Magn Reson Imaging 24:41–51
Lupo JM, Cha S, Chang SM, Nelson SJ (2007) Analysis of metabolic indices in regions of abnormal perfusion in patients with high-grade glioma. AJNR Am J Neuroradiol 28:1455–1461
Lehmann P, Saliou G, De Marco G et al (2011) Cerebral peritumoral oedema study: does a single dynamic MR sequence assessing perfusion and permeability can help to differentiate glioblastoma from metastasis? Eur J Radiol 81:522-527
Acknowledgments
First authors: T. Smets and T. M. Lawson contributed equally to the manuscript. Last authors: A. Jankovski and C. Raftopoulos contributed equally to the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smets, T., Lawson, T.M., Grandin, C. et al. Immediate post-operative MRI suggestive of the site and timing of glioblastoma recurrence after gross total resection: a retrospective longitudinal preliminary study. Eur Radiol 23, 1467–1477 (2013). https://doi.org/10.1007/s00330-012-2762-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-012-2762-1