Abstract
Objective
To evaluate the effect of monophasic combined oral contraceptive pill (COCP) and menstrual cycle phase in healthy young women on white matter (WM) organization using diffusion tensor imaging (DTI).
Methods
Thirty young women were included in the study; 15 women used COCP and 15 women had a natural cycle. All subjects underwent DTI magnetic resonance imaging during the follicular and luteal phase of their cycle, or in different COCP cycle phases. DTI parameters were obtained in different WM structures by performing diffusion tensor fibre tractography. Fractional anisotropy and mean diffusivity were calculated for different WM structures. Hormonal plasma concentrations were measured in peripheral venous blood samples and correlated with the DTI findings.
Results
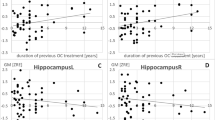
We found a significant difference in mean diffusivity in the fornix between the COCP and the natural cycle group. Mean diffusivity values in the fornix were negatively correlated with luteinizing hormone and estradiol blood concentrations.
Conclusion
An important part in the limbic system, the fornix, regulates emotional processes. Differences in diffusion parameters in the fornix may contribute to behavioural alternations related to COCP use. This finding also suggests that the use of oral contraceptives needs to be taken into account when designing DTI group studies.
Key Points
• Diffusion tensor MRI offers new insights into brain white matter microstructure.
• The effects of oral hormonal contraception were examined in young women.
• Diffusion tensor images and hormone blood concentrations were evaluated.
• Women using hormonal contraception demonstrated higher mean diffusivity in the fornix.
• These changes may contribute to behavioural alternations related to contraception use.



Similar content being viewed by others
Abbreviations
- AD:
-
axial diffusivity
- CSF:
-
cerebrospinal fluid
- COCP:
-
combined oral contraceptive pill
- DKI:
-
diffusion kurtosis imaging
- DTI:
-
diffusion tensor imaging
- EPI:
-
echo planar imaging
- FA:
-
fractional anisotropy
- FOV:
-
field of view
- FSH:
-
follicle-stimulating hormone
- GM:
-
grey matter
- LH:
-
luteinizing hormone
- MD:
-
mean diffusivity
- RD:
-
radial diffusivity
- ROI:
-
region of interest
- SD:
-
standard deviation
- VBA:
-
voxel-based analysis
- WM:
-
white matter
References
Boron W, Boulpaep E (2005) Medical physiology. Elsevier, Philadelphia
Naftolin F, MacLusky NJ, Leranth CZ, Sakamoto HS, Garcia-Segura LM (1988) The cellular effects of estrogens on neuroendocrine tissues. J Steroid Biochem 30:195–207
Naftolin F, Garcia-Segura LM, Horvath TL et al (2007) Estrogen-induced hypothalamic synaptic plasticity and pituitary sensitization in the control of the estrogen-induced gonadotrophin surge. Reprod Sci 14:101–116
Blumenfeld-Katzir T, Pasternak O, Dagan M, Assaf Y (2011) Diffusion MRI of structural brain plasticity induced by a learning and memory task. PLoS One 6:e20678
Protopopescu X, Pan H, Altemus M et al (2005) Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. Proc Natl Acad Sci U S A 102:16060–16065
Goldstein JM, Jerram M, Poldrack R et al (2005) Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci 25:9309–9316
Konrad C, Engelien A, Schoning S et al (2008) The functional anatomy of semantic retrieval is influenced by gender, menstrual cycle, and sex hormones. J Neural Transm 115:1327–1337
Guapo VG, Graeff FG, Zani AC, Labate CM, dos Reis RM, Del-Ben CM (2009) Effects of sex hormonal levels and phases of the menstrual cycle in the processing of emotional faces. Psychoneuroendocrinology 34:1087–1094
Rupp HA, James TW, Ketterson ED, Sengelaub DR, Janssen E, Heiman JR (2009) Neural activation in the orbitofrontal cortex in response to male faces increases during the follicular phase. Horm Behav 56:66–72
Zhu X, Wang X, Parkinson C, Cai C, Gao S, Hu P (2010) Brain activation evoked by erotic films varies with different menstrual phases: an fMRI study. Behav Brain Res 206:279–285
Ossewaarde L, Hermans EJ, van Wingen GA et al (2010) Neural mechanisms underlying changes in stress-sensitivity across the menstrual cycle. Psychoneuroendocrinology 35:47–55
Basser PJ, Mattiello J, LeBihan D (1994) Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B 103:247–254
Basser PJ (1995) Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed 8:333–344
Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A (2000) In vivo fibre tractography using DT-MRI data. Magn Reson Med 44:625–632
Beaulieu C (2002) The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed 15:435–455
Herting MM, Maxwell EC, Irvine C, Nagel BJ (2011) The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cereb Cortex. doi:10.1093/cercor/bhr246
Leemans A, Jones DK (2009) The B-matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med 61:1336–1349
Chang LC, Jones DK, Pierpaoli C (2005) RESTORE: robust estimation of tensors by outlier rejection. Magn Reson Med 53:1088–1095
Leemans A, Jeurissen B, Sijbers J, Jones DK (2009) ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. Proc Intl Soc Mag Reson Med 17:3536
Engvig A, Fjell AM, Westlye LT, et al (2011) Memory training impacts short-term changes in aging white matter: a longitudinal diffusion tensor imaging study. Hum Brain Mapp. doi:10.1002/hbm.21370
Lazar M (2010) Mapping brain anatomical connectivity using white matter tractography. NMR Biomed 23:821–835
Cheng H, Wang Y, Sheng J et al (2012) Optimization of seed density in DTI tractography for structural networks. J Neurosci Methods 203:264–272
Tournier JD, Mori S, Leemans A (2011) Diffusion tensor imaging and beyond. Magn Reson Med 65:1532–1556
Vos SB, Jones DK, Jeurissen B, Viergever MA, Leemans A (2012) The influence of complex white matter architecture on the mean diffusivity in diffusion tensor MRI of the human brain. NeuroImage 59:2208–2216
Naninck EF, Lucassen PJ, Bakker J (2011) Sex differences in adolescent depression: do sex hormones determine vulnerability? J Neuroendocrinol 23:383–392
Song SK, Yoshino J, Le TQ et al (2005) Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage 26:132–140
Beaulieu C (2009) The biological basis of diffusion anisotropy. In: Johansen-Berg H, Behrens TEJ (eds) Diffusion MRI: from quantitative measurement to in-vivo neuroanatomy. Elsevier, London, pp 105–126
Metzler-Baddeley C, O'Sullivan MJ, Bells S, Pasternak O, Jones DK (2011) How and how not to correct for CSF-contamination in diffusion MRI. Neuroimage 59:1394–1403
Snook L, Plewes C, Beaulieu C (2007) Voxel based versus region of interest analysis in diffusion tensor imaging of neurodevelopment. NeuroImage 34:243–252
Van Hecke W, Leemans A, D’Agostino E et al (2007) Nonrigid coregistration of diffusion tensor images using a viscous fluid model and mutual information. IEEE Trans Med Imaging 26:1598–1612
Van Hecke W, Sijbers J, D'Agostino E et al (2008) On the construction of an inter-subject diffusion tensor magnetic resonance atlas of the healthy human brain. NeuroImage 43:69–80
Veraart J, Van Hecke W, Sijbers J (2011) Constrained maximum likelihood estimation of the diffusion kurtosis tensor using a Rician noise model. Magn Reson Med 66:678–686
Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C (2008) Microstructural maturation of the human brain from childhood to adulthood. NeuroImage 40:1044–1055
Verhoeven JS, Sage CA, Leemans A et al (2010) Construction of a stereotaxic DTI atlas with full diffusion tensor information for studying white matter maturation from childhood to adolescence using tractography-based segmentations. Hum Brain Mapp 31:470–486
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Bondt, T., Van Hecke, W., Veraart, J. et al. Does the use of hormonal contraceptives cause microstructural changes in cerebral white matter? Preliminary results of a DTI and tractography study. Eur Radiol 23, 57–64 (2013). https://doi.org/10.1007/s00330-012-2572-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-012-2572-5