Abstract
Objectives
To compare mesoscopic epi-fluorescence tomography (MEFT) and EPRI-illumination reflectance imaging (EPRI) for quantitative tumour size assessment in mice.
Methods
Tumour xenografts of green/red fluorescent protein (GFP/RFP)-expressing colon cancer cells were measured using MEFT, EPRI, ultrasound (US) and micro computed tomography (μCT) at day 14 post-injection (n = 6). Results from MEFT and EPRI were correlated with each other and with US and μCT (reference methods). Tumour volumes were measured ex vivo by GFP and RFP fluorescence imaging on cryoslices and compared with the in vivo measurements.
Results
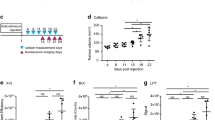
High correlation and congruency were observed between MEFT, US and μCT (MEFT/US: GFP: r 2 = 0.96; RFP: r 2 = 0.97, both P < 0.05; MEFT/μCT: GFP: r 2 = 0.93; RFP: r 2 = 0.90; both P < 0.05). Additionally, in vivo MEFT data were highly correlated and congruent with ex vivo cryoslice imaging results (GFP: r 2 = 0.96; RFP: r 2 = 0.99; both P < 0.05). In comparison, EPRI significantly overestimated tumour volumes (P < 0.05), although there was a significant correlation with US and μCT (EPRI/US: GFP: r 2 = 0.95; RFP: r 2 = 0.94; both P < 0.05; EPRI/μCT GFP: r 2 = 0.86; RFP: r 2 = 0.86; both P < 0.05).
Conclusions
Fluorescence distribution reconstruction using MEFT affords highly accurate three-dimensional (3D) tumour volume data showing superior accuracy compared to EPRI. Thus, MEFT is a very suitable technique for quantitatively assessing fluorescence distribution in superficial tumours at high spatial resolution.
Key Points
• Mesoscopic epi-fluorescence tomography (MEFT) is an important new molecular imaging technique.
• MEFT allows accurate size determination of superficial tumours with high resolution.
• MEFT is a suitable technique for longitudinal assessment of tumour growth.
• MEFT allows 3D reconstruction and quantification of fluorescence distributions.






Similar content being viewed by others
References
Graham KC, Wirtzfeld LA, MacKenzie LT et al (2005) Three-dimensional high-frequency ultrasound imaging for longitudinal evaluation of liver metastases in preclinical models. Cancer Res 65:5231–5237
Wessels JT, Busse AC, Mahrt J, Dullin C, Grabbe E, Mueller GA (2007) In vivo imaging in experimental preclinical tumor research–a review. Cytometry 71:542–549
Weissleder R, Pittet MJ (2008) Imaging in the era of molecular oncology. Nature 452:580–589
Hoffman RM (2005) The multiple uses of fluorescent proteins to visualize cancer in vivo. Nat Rev Cancer 5:796–806
Abou-Elkacem L, Gremse F, Barth S, Hoffman RM, Kiessling F, Lederle W (2011) Comparison of μCT, MRI and optical reflectance imaging for assessing the growth of GFP/RFP-expressing tumors. Anticancer Res 31:2907–2913
Ntziachristos V, Ripoll J, Wang LV, Weissleder R (2005) Looking and listening to light: the evolution of whole-body photonic imaging. Nat Biotech 23:313–320
Björn S, Ntziachristos V, Schulz R (2010) Mesoscopic epifluorescence tomography: reconstruction of superficial and deep fluorescence in highly-scattering media. Opt Express 18:8422–8429
Björn S, Englmeier KH, Ntziachristos V, Schulz R (2011) Reconstruction of fluorescence distribution hidden in biological tissue using mesoscopic epifluorescence tomography. J Biomed Opt 16:046005
Jiang P, Yamauchi K, Yang M et al (2006) Tumor cells genetically labeled with GFP in the nucleus and RFP in the cytoplasm for imaging cellular dynamics. Cell Cycle 5:1198–1201
Yamamoto N, Jiang P, Yang M et al (2004) Cellular dynamics visualized in live cells in vitro and in vivo by differential dual-color nuclear-cytoplasmic fluorescent-protein expression. Cancer Res 64:4251–4256
Hoffman RM, Yang M (2006) Subcellular imaging in the live mouse. Nat Protoc 1:775–782
Cheung AMY, Brown AS, Hastie LA et al (2005) Three-dimensional ultrasound biomicroscopy for xenograft growth analysis. Ultrasound Med Biol 31:865–870
Sarantopoulos A, Themelis G, Ntziachristos V (2010) Imaging the bio-distribution of fluorescent probes using multispectral epi-illumination cryoslicing imaging. Mol Imaging Biol 13:874–885
Ntziachristos V (2010) Going deeper than microscopy: the optical imaging frontier in biology. Nat Methods 7:603–614
Vinegoni C, Pitsouli C, Razansky D, Perrimon N, Ntziachristos V (2007) In vivo imaging of Drosophila melanogaster pupae with mesoscopic fluorescence tomography. Nat Methods 5:45–47
Weissleder R, Ntziachristos V (2003) Shedding light onto live molecular targets. Nat Med 9:123–128
Crane LMA, Themelis G, Pleijhuis RG et al (2011) Intraoperative multispectral fluorescence imaging for the detection of the sentinel lymph node in cervical cancer: a novel concept. Mol Imaging Biol 13:1043–1049
van Dam GM, Themelis G, Crane LMA et al (2011) Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nat Med 17:1315–1319
Katz MH, Takimoto S, Spivack D, Moossa AR, Hoffman RM, Bouvet M (2003) A novel red fluorescent protein orthotopic pancreatic cancer model for the preclinical evaluation of chemotherapeutics. J Surg Res 113:151–160
Deliolanis NC, Wurdinger T, Pike L et al (2011) In vivo tomographic imaging of red-shifted fluorescent proteins. Biomed Opt Express 2:887–900
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (KI 1072/2-1) and by High Tech NRW (ForSaTum).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Lotfi Abou-Elkacem and Saskia Björn contributed equally to this article.
Rights and permissions
About this article
Cite this article
Abou-Elkacem, L., Björn, S., Doleschel, D. et al. High accuracy of mesoscopic epi-fluorescence tomography for non-invasive quantitative volume determination of fluorescent protein-expressing tumours in mice. Eur Radiol 22, 1955–1962 (2012). https://doi.org/10.1007/s00330-012-2462-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-012-2462-x