Abstract
Objectives
Patients with prosthetic heart valves may require assessment for coronary artery disease. We assessed whether valve artefacts hamper coronary artery assessment by multidetector CT.
Methods
ECG-gated or -triggered CT angiograms were selected from our PACS archive based on the presence of prosthetic heart valves. The best systolic and diastolic axial reconstructions were selected for coronary assessment. Each present coronary segment was scored for the presence of valve-related artefacts prohibiting coronary artery assessment. Scoring was performed in consensus by two observers.
Results
Eighty-two CT angiograms were performed on a 64-slice (n = 27) or 256-slice (n = 55) multidetector CT. Eighty-nine valves and five annuloplasty rings were present. Forty-three out of 1160 (3.7%) present coronary artery segments were non-diagnostic due to valve artefacts (14/82 patients). Valve artefacts were located in right coronary artery (15/43; 35%), left anterior descending artery (2/43; 5%), circumflex artery (14/43; 32%) and marginal obtuse (12/43; 28%) segments. All cobalt-chrome containing valves caused artefacts prohibiting coronary assessment. Biological and titanium-containing valves did not cause artefacts except for three specific valve types.
Conclusions
Most commonly implanted prosthetic heart valves do not hamper coronary assessment on multidetector CT. Cobalt-chrome containing prosthetic heart valves preclude complete coronary artery assessment because of severe valve artefacts.
Key Points
• Most commonly implanted prosthetic heart valves do not hamper coronary artery assessment
• Prosthetic heart valve composition determines the occurrence of prosthetic heart valve-related artefacts
• Björk–Shiley and Sorin tilting disc valves preclude diagnostic coronary artery segment assessment
Similar content being viewed by others
Introduction
The introduction of ECG-gated and triggered multidetector computed tomography (MDCT) technology has resulted in new diagnostic cardiac applications, most noticeably coronary artery assessment [1]. Recently, MDCT has also shown potential for the evaluation of prosthetic heart valve (PHV) (dys)function by providing complementary diagnostic information to echocardiography and fluoroscopy, especially in patients with suspected PHV obstruction and PHV endocarditis [2–6].
Besides evaluation of the exact cause of PHV dysfunction, MDCT may have complementary clinical value for the surgical planning in patients considered for reoperation after previous PHV implantation. MDCT can be used to evaluate the presence of coronary artery disease (CAD) and the patency of present bypass grafts to serve as an alternative for classical coronary angiography, and the distance between the sternum and the right ventricle can be measured [7]. Patients with a normal functioning PHV may also be candidates for CT assessment of the coronary arteries in newly developed angina.
Each PHV type has its own specific imaging characteristics on CT [8, 9]. Most PHVs have limited artefacts but at present little is known about which PHV types and positions induce artefacts to such an extent that they prohibit coronary assessment on MDCT. Knowledge of which PHV types and positions prohibit coronary assessment is clinically important to determine whether adequate CT assessment of coronary segments is to be expected or that the patient should be referred for conventional coronary angiography straight away. The purpose of this study was to determine which PHV types induce artefacts that hamper coronary artery segment assessment on MDCT in a cohort of patients with a PHV in whom an ECG-gated or -triggered PHV had been performed.
Materials and methods
CT angiogram selection
We reviewed all ECG-gated or prospectively triggered PHV CT angiograms (CTAs) performed in the University Medical Center Utrecht and Academic Medical Center Amsterdam between 2003 and September 2011 for the presence of PHVs that were imaged in both systolic and diastolic phase. We excluded CTAs that included only systolic or diastolic imaging, were performed on 16-slice MDCT, non-contrast enhanced imaging as well as CTAs in patients enrolled in an ongoing prospective diagnostic cross-sectional study on CT PHV assessment. Furthermore, patients with multiple CTA examinations were included only once. Patients with a concomitant mitral or tricuspid annuloplasty ring were included.
CTAs were performed on 64 or 256 slice MDCT systems (Brilliance 64 and iCT, Philips Medical Systems, Cleveland, Ohio). Contrast agents [Ultravist (iopromide) - 300 mg I/mL, Bayer Schering Pharma AG, Berlin, Germany or Iomeron (iomeprol) - 400 mg I/mL, Bracco UK Limited, London, United Kingdom] were administered in all patients. Cardiac CTAs were performed with a triphasic or dual phasic contrast injection protocol. The triphasic protocol started with a 100% contrast injection (phase 1) followed by a 30%/70% contrast/saline mixture (phase 2), and concluded with a saline flush (phase 3). Contrast volumes were adjusted for the patients body weight with an iodine flow of 1.6 g/s for patients <70 kilogram (kg), 1.8 g/s for patients 70–85 kg and 2.0 g/s for patients >85 kg. The dual phase contrast protocol consisted of a contrast injection of 100 millilitre (mL) followed by a saline flush. For aortic CTAs, a fixed bolus of 100 ml was followed by a saline flush of 50 ml. In general, injection flow was set to 5–6 ml/s. The CTA selection resulted in the inclusion of 82 patients (48 males, 34 females) with 94 PHVs and annuloplasty rings. The mean age of our study population was 58 ± 14 years (mean ± SD). All 82 patients underwent CTA examinations on 64-slice (n = 27) or 256-slice (n = 55) MDCT systems. The indications for CTA examinations were: PHV dysfunction (n = 47), aortic aneurysm evaluation (n = 17), aortic dissection (n = 8), coronary assessment (n = 3), cardiac other (n = 5) and other (n = 2). In four of 82 patients (5%) beta-blockers were administered to lower the heart rate. Nitroglycerin was not routinely administered in our patients.
CT data were retrieved from the PACS archive of the Radiology department and sent to a dedicated workstation for image analysis. Heart rate during imaging was obtained from the CT data. DLP values were obtained if dose information was available. DLP values were converted to radiation exposure (mSv) by using 0.017 for cardiac CTAs, and 0.016 (mean of 0.017 for the chest and 0.015 for the abdomen) for thoraco-abdominal aortic CTAs as conversion factor. Patient and PHV data were obtained from the patient’s medical files. The study was performed under a waiver from the institutional reviewing board. Data on the image quality of the PHV itself in a number of these patients have been previously published [9].
Image analysis
Assessment of the MDCT was performed on a dedicated workstation (Extended Brilliance Workstation, Philips Healthcare, Best, The Netherlands). The best systolic and diastolic image phase in the retrospectively gated or prospectively triggered CT dataset for coronary assessment was selected. Coronary arteries were assessed according to the 17-segment modified American Heart Association classification [10] on the axial images (Fig. 1).
Coronary artery segments according to the American Heart Association classification [10]. Ao = Aorta; RCA = Right Coronary Artery; LCA = Left Coronary Artery; LM = Left Main branch; LAD = Left Anterior Descending Coronary Artery; LCX = Left Circumflex Coronary Artery
First, each coronary segment was scored as present or absent. Absent was defined as no visible segment, but sufficient high image quality to exclude non-visibility of other causes or a segment not included in the imaging range. For present segments, the image quality was scored on a three-point scale (1 = non-diagnostic, 2 = acceptable, 3 = good). The criteria for the different scores were formulated as follows: (1) non-diagnostic: segment details not sufficient visualised to perform diagnostic assessment; (2) acceptable: adequate segment details with limited artefacts; and (3) good: perfect segment details without artefacts. Reasons for non-diagnostic segments were classified as: valve- or ring annuloplasty-related artefacts, or other (e.g. motion, small vessel size, low contrast enhancement and pacemaker lead artefacts). Scoring was performed in consensus by two observers (JH and RB) with 2 years and 5 years experience in CT coronary assessment, respectively.
Data analysis
Data analysis was restricted to descriptive statistics. Data were presented as means ± standard deviation (SD) for continuous data with a parametric data distribution. Categorical data were presented in total numbers and/or percentages. The presence of PHV-related artefacts precluding coronary artery segment assessment was determined for the different PHV types and PHV positions. These PHV-related artefacts are presented per coronary artery segment.
Results
Mean heart rate during the 82 CTA examinations was 73 ± 18 beats per minute (bpm). In 15 of the 82 patients (18%), the heart rate during CT data acquisition was missing. Mean heart rates were 73 ± 18 bpm and 72 ± 19 bpm for the mechanical PHV group and biological PHV group, respectively. Sixty of 82 (73%) CT acquisitions were performed as a dedicated cardiac CTA including 51 retrospectively ECG-gated CTAs (120 kV, ≥600 mAs) and nine prospectively triggered CTAs (120 kV, 200–250 mAs); and 22/82 (27%) CTAs as a retrospectively ECG-gated CTA of the thoraco-(abdominal) aorta with a lower tube current (200–400 mAs). Radiation dose information was available for 42/82 patients (51%). Mean radiation dose for retrospectively ECG-gated cardiac CTAs was 15.6 ± 5.5 mSv, and for thoraco-abdominal aortic CTAs 17.6 ± 4.2 mSv. Ninety-four PHVs and annuloplasty rings of 14 different PHV and annuloplasty ring types were present: 68 mechanical PHVs (72%), 21 biological PHVs (22%), and 5 annuloplasty rings (5%) (mitral n = 4 and tricuspid n = 1). PHVs and annuloplasty rings were positioned in the aortic (n = 73; 78%), mitral (n = 18; 19%), pulmonary (n = 1; 1%) and tricuspid (n = 2; 2%) positions (Table 1).
PHV-related artefacts interfering with coronary artery assessment on MDCT
In total 17/94 (18%) PHVs and annuloplasty rings induced artefacts prohibiting assessment of at least one coronary segment (Table 1). Valve-related artefacts per coronary segment are shown in Tables 2 and 3.
A theoretical total of 1394 coronary segments (82 patients × 17 segments) were available for analysis. Two hundred and thirty-four of the 1394 segments (17%) were scored absent. In total 1160 coronary segments were present for assessment. Three-hundred and six of 1160 coronary segments (26%) demonstrated a non-diagnostic image quality, 538/1160 (46%) had acceptable image quality; and in 316/1160 (27%) image quality was good. The reasons for non-diagnostic image quality were PHV-related artefacts (n = 43; 14%), motion (n = 60 segments; 20%), vessel size (n = 192; 63%); and pacemaker artefacts (n = 11; 4%) (Fig. 2). In patients with more than one PHV implanted, no coronary segments were non-assessable owing to artefacts originating from both valves.
None of the biological PHVs (0/21; 0%) and annuloplasty rings (0/5; 0%), and none of the ON-X (0/6; 0%) and Medtronic Hall (0/12; 0%) mechanical PHVs caused artefacts prohibiting coronary assessment.
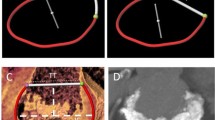
In the aortic position, St Jude (2/8; 25%) and Sorin (3/5; 60%) bileaflet PHVs caused artefacts prohibiting coronary assessment, mainly of the proximal right coronary artery (RCA). The Björk–Shiley (n = 3; 100%) and Sorin tilting disc PHVs (n = 1; 100%) caused severe artefacts in RCA segments. Moreover, the Björk–Shiley tilting disc also caused artefacts in the left circumflex (LCX) and obtuse marginal (MO) branches (Table 2). The commonly implanted PHVs (Carbomedics bileaflet (n = 21; 0%) and Medtronic Hall tilting disc (n = 9; 0%) demonstrated no PHV-related artefacts in the aortic position (Tables 1 and 2). Figures 3 and 4 illustrate aortic PHVs with and without PHV-related artefacts in the RCA.
In the mitral position, Carbomedics (1/3; 33%), St Jude (3/4; 75%) and the Duromedics (1/1; 100%) bileaflet PHVs caused artefacts prohibiting assessment of at least one coronary segment in the LCX and MO branches. Both Björk–Shiley and the Sorin tilting disc PHVs caused severe artefacts in LCX and MO segments. The Björk–Shiley tilting disc also caused severe artefacts in RCA segments. Affected segments are detailed in Table 3. Figure 5 illustrates mitral PHVs with and without artefacts in the LCX. In the pulmonary position, the Sorin bileaflet PHV (n = 1; 100%) demonstrated PHV-related artefacts in RCA segment 2. In the tricuspid position, ON-X bileaflet PHV (n = 1) and tricuspid annuplasty ring (n = 1) showed no PHV-related artefacts (Table 3).
PHV related artefacts in different imaging phases
In all patients, coronary artery segments were assessed in the best systolic and diastolic imaging phase. In the aortic position, four patients (5%) demonstrated PHV-related artefacts precluding diagnostic coronary assessment of RCA segment 1 only in diastolic (n = 2; 50%) or systolic (n = 2; 50%) phase. In the mitral position, one patient (6%) had only PHV-related artefacts in the diastolic phase in the LCX and MO branches.
In summary, PHV-related artefacts were present in RCA segments (1, 2 and 3), left anterior descending artery (LAD) segment 8, LCX segments (11 and 13), and MO branches (12 and 14). The close relationship between RCA and aortic PHV, and LCX and mitral PHV is illustrated in Fig. 6. The assessment of the remaining coronary segments (including the left main branch, Fig. 7) was not disturbed by PHV-related artefacts (Fig. 1, Tables 2 and 3).
Discussion
The principle findings of our study are: (1) the diagnostic assessment of most coronary artery segments in patients with PHVs was not hampered by PHV-related artefacts, (2) Björk–Shiley tilting disc PHVs, Sorin tilting disc PHVs and Duromedics bileaflet PHVs precluded complete diagnostic assessment of coronary artery segments; and (3) ON-X and Medtronic Hall PHVs, biological PHVs and annuloplasty rings never hampered coronary artery assessment by MDCT.
The PHV-related artefacts on MDCT images seem to be more dependent on PHV composition than on the PHV design (bileaflet, tilting disc and biological valves) (Table 4) [8, 9]. As far as the material of the PHV is concerned our study showed notable differences.
Björk–Shiley and Sorin tilting disc PHVs as well as Duromedics bileaflet PHVs demonstrated severe artefacts which precluded diagnostic coronary assessment of RCA, LCX and the distal segment of the LAD, dependent on the PHV position. This finding is supported by previous studies that described that these PHV types as being associated with valve-related artefacts that also prohibit the evaluation of the PHV itself on MDCT [8, 9, 11, 12]. The severe artefacts of the Björk–Shiley, Sorin tilting disc and Duromedics bileaflet PHVs were caused by the cobalt-chrome alloy that is present in these valves. Therefore, MDCT is not suitable for the assessment of the coronary arteries in patients who have one of these PHVs implanted. The Saint Jude PHV, consisting of a nickel alloy, produced fewer artefacts but still enough to preclude assessment of a limited number of coronary artery segments in the right coronary artery and circumflex territory (segments 1, 13 and 14). The other segments did not suffer from artefacts that precluded assessment. The Saint Jude PHV is one of the most commonly implanted PHV types worldwide and therefore one of the most likely PHVs to be encountered. The possibility of the non-diagnostic image quality of the above-mentioned segments should be kept in mind when performing cardiac CT in these patients.
In general, Carbomedics, ON-X and Medtronic Hall mechanical PHVs caused no PHV-related artefacts hampering coronary artery assessment. These PHVs are mainly composed of titanium alloys that are associated with only limited artefacts on MDCT [8].
The biological PHVs and annuloplasty rings produced fewer PHV artefacts than mechanical PHVs on MDCT. Some biological PHVs have a radiopaque frame that supports the valve leaflets, but this does not generally induce many artefacts [2, 9]. In our study, no PHV-related artefacts that interfered with diagnostic coronary assessment were found in biological PHVs or annuloplasty rings.
Prosthetic heart valves in the aortic position may show mainly artefacts in the proximal RCA (segments 1 and 2) because of the close relationship between the aortic PHV and the proximal RCA. Interestingly, the left main branch, which also has a close relationship with the aortic PHV, did not show any PHV-related artefacts. The reason for the absence of PHV-related artefacts in this segment may be the angulation of the PHV with respect to the X-ray beam of the gantry.
In the mitral position, PHV-related artefacts occurred in the LCX and MO branches. The close relationship between these segments and the mitral PHV position is a likely explanation for the presence of the PHV-related artefacts in these specific segments.
Limitations
Our study contains many different PHV types. A few PHV types are only represented in small numbers. However the most commonly implanted PHVs (Carbomedics, St Jude, Medtronic Hall and the Perimount biological PHVs) are present in considerable numbers. The mean heart rate of 73 ± 18 bpm is relatively high for CT coronary assessment because 79 of 82 CTAs (96%) were performed for other clinical indications without a specific heart rate reduction. High heart rates may cause considerable motion artefacts that influence diagnostic CAD assessment. However, the specific interest of this study was to address the question: do PHV-related artefacts disturb coronary assessment? Other reports emphasised the importance of an optimal heart rate and the restricted diagnostic value of MDCT in the detection of CAD in distal coronary segments [13]. To optimize coronary image quality, beta-blockers and nitroglycerin should be routinely administered in patients without contraindications for these drugs. In this study, CT coronary angiography was not compared with conventional coronary angiography. Further prospective studies are required to determine the diagnostic accuracy of CT coronary angiography in patients after PHV implantation. However, as most commonly implanted PHV types generate only limited artefacts, we would expect the diagnostic accuracy to be close to published results in patients who have not yet undergone PHV implantation [13]. Consensus reading was performed. Interobserver variability has to be investigated in further studies to validate the PHV-related artefacts scoring system. Axial CT images only were assessed for the presence of PHV-related artefacts. Multiplanar reconstructions were not separately assessed because PHV-related artefacts were present in both axial and multiplanar reconstructions.
In conclusion, the most commonly implanted PHVs do not cause artefacts that prohibit coronary artery assessment of at least one coronary segment by MDCT. Carbomedics, Medtronic Hall and ON-X mechanical PHVs, bioprosthesis, and annuloplasty rings virtually never hamper coronary artery assessment by MDCT. However, in patients with a Björk–Shiley or Sorin tilting PHV, coronary artery assessment by MDCT is virtually always hampered by PHV induced artefacts.
References
Meijboom WB, Meijs MF, Schuijf JD et al (2008) Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol 52:2135–2144
Chenot F, Montant P, Goffinet C et al (2010) Evaluation of anatomic valve opening and leaflet morphology in aortic valve bioprosthesis by using multidetector CT: comparison with transthoracic echocardiography. Radiology 255:377–385
Feuchtner GM, Stolzmann P, Dichtl W et al (2009) Multislice computed tomography in infective endocarditis: comparison with transesophageal echocardiography and intraoperative findings. J Am Coll Cardiol 53:436–444
Habets J, Budde RP, Symersky P et al (2011) Diagnostic evaluation of left-sided prosthetic heart valve dysfunction. Nat Rev Cardiol 8:466–478
Symersky P, Budde RP, de Mol BA et al (2009) Comparison of multidetector-row computed tomography to echocardiography and fluoroscopy for evaluation of patients with mechanical prosthetic valve obstruction. Am J Cardiol 104:1128–1134
Tsai IC, Lin YK, Chang Y et al (2009) Correctness of multi-detector-row computed tomography for diagnosing mechanical prosthetic heart valve disorders using operative findings as a gold standard. Eur Radiol 19:857–867
Bonow RO, Carabello BA, Chatterjee K et al (2008) 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 52:e1–e142
Symersky P, Budde RP, Prokop MA et al (2011) Multidetector-row computed tomography imaging characteristics of mechanical prosthetic valves. J Heart Valve Dis 20:216–222
Habets J, Symersky P, van Herwerden LA et al (2011) Prosthetic heart valve assessment with multidetector-row CT: imaging characteristics of 91 valves in 83 patients. Eur Radiol 21:1390–1396
Austen WG, Edwards JE, Frye RL et al (1975) A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 51:5–40
LaBounty TM, Agarwal PP, Chughtai A et al (2009) Evaluation of mechanical heart valve size and function with ECG-gated 64-MDCT. Am J Roentgenol 193:389–396
Konen E, Goitein O, Feinberg MS et al (2008) The role of ECG-gated MDCT in the evaluation of aortic and mitral mechanical valves: initial experience. Am J Roentgenol 191:26–31
Meijboom WB, Mollet NR, Van Mieghem CA et al (2006) Pre-operative computed tomography coronary angiography to detect significant coronary artery disease in patients referred for cardiac valve surgery. J Am Coll Cardiol 48:1658–1665
Acknowledgement
We thank K.A. van Rijnbach (Department of Radiology, University Medical Center Utrecht) for her help with the final edition of the Figures and I.G. Janssen (Department of Multimedia Production, University Medical Center Utrecht) for designing Figure 1 and 6. The present study was supported by a grant from the Netherlands Heart Foundation [Grant number 2009B014]
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Habets, J., van den Brink, R.B.A., Uijlings, R. et al. Coronary artery assessment by multidetector computed tomography in patients with prosthetic heart valves. Eur Radiol 22, 1278–1286 (2012). https://doi.org/10.1007/s00330-011-2360-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-011-2360-7