Abstract
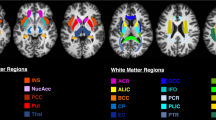
Our purpose was to reveal potential regional variations in water molecular diffusivity within each cerebral hemisphere and across the right and left hemispheres. Diffusion-weighted images of 44 healthy right-handed adult male subjects were obtained using a diffusion tensor imaging sequence. Mean diffusivity (MD) values in subcortical white matter (WM) within 39 regions in each hemisphere were measured using an automated method. Intrahemispheric comparisons of MDs in subcortical WM were performed among six brain regions (frontal, parietal, occipital and temporal lobes and pre- and postcentral gyri). Interhemispheric comparisons of MDs were performed between the right and left counterparts of the 39 regions. In both hemispheres, diffusivity in the precentral gyrus was lower than those in other regions, while diffusivity in the parietal lobe was higher than others. MD asymmetry in which the left was lower than the right was found in the parietal lobe, middle occipital gyrus, and medial and orbital aspects of the frontal lobe. The converse asymmetry was revealed in the frontal operculum, supplementary motor cortex, temporal lobe, limbic cortices, precuneus and cuneus. Our results revealed significant intra- and interhemispheric regional variations in MD in subcortical WM, which may be related to different densities of axons and myelin sheaths.

Similar content being viewed by others
References
Brodmann K (1909) Vergleichende Localisationlehre der Grosshirnrinde. Springer-Verlag, Leipzig
von Economo C, Koskinas GH (1925) Die Cytoarchitektonic der Hirnrinde des Erwachsenen Menschen. Springer Verlag, Wien-Berlin
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97:11050–11055
Sigalovsky IS, Fischl B, Melcher JR (2006) Mapping an intrinsic MR property of gray matter in auditory cortex of living humans: a possible marker for primary cortex ad hemispheric differences. Neuroimage 32:1524–1537
Georgiades CS, Itoh R, Golay X et al (2001) MR imaging of the human brain at 1.5 T: regional variations in transverse relaxation rates in he cerebral cortex. AJNR Am J Neuroradiol 22:1732–1737
Geschwind N, Levitsky W (1968) Human brain: left-right asymmetry in temporal speech region. Science 161:186–187
Stenmetz H, Rademacher J, Huang YX et al (1989) Cerebral asymmetry: MR planimetry of the human planum temporale. J Comput Assist Tomogr 13:996–1005
Chui HD, Damasio AR (1980) Human cerebral asymmetries evaluated by computerized tomography. J Neurol Neurosurg Psychiatry 43:873–878
Good CD, Johnsrude I, Ashburner J et al (2001) Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage 14:685–700
Watkins KE, Paus T, Lerch JP et al (2001) Structural asymmetry in the human brain: a voxel-based statistical analysis of 142 MRI scans. Cerebral Cortex 11:868–877
Shibata DK (2007) Difference in brain structure in deaf persons on MR imaging studied with voxel-based morphomertry. AJNR Am J Neuroradiol 28:243–249
Le Bihan D, Breton E, Lallemand D et al (1986) MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 161:401–1407
Basser PJ, Pierpaoli C (1996) Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 111:209–219
Pierpaoli C, Jezzard P, Basser PJ et al (1996) Diffusion tensor MR imaging of the human brain. Radiology 201:637–648
Buechel C, Raedler T, Sommer M et al (2004) White matter asymmetry in he human brain: a diffusion tensor MRI study. Cerebral Cortex 14:945–951
Vernooij MW, Smits M, Wielopolski PA et al (2007) Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right- and left-handed healthy subjects: a combined fMRI and DTI study. Neuroimage 35:1064–1076
Naganawa S, Sato K, Katagiri T et al (2003) Regional ADC values of the normal brain: differences due to age, gender, and laterality. Eur Radiol 13:6–11
Yoshiura T, Mihara F, Tanaka A et al (2005) Novel method to estimate and display cerebral cortical degeneration using diffusion-weighted magnetic resonance imaging. Magn Reson Med 54:455–459
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Zung WW (1965) A self-rating depression scale. Arch Gen Psychiatry 12:63–70
Alexander AL, Lee JE, Wu YC et al (2006) Comparison of diffusion tensor imaging measurements at 3.0 T versus 1.5 T with and without parallel imaging. Neuroimaging Clin N Am 16:299–309
Kwong KK, McKinstry RC, Chien D et al (1991) CSF-suppressed quantitative single-shot diffusion imaging. Magn Reson Med. 21:157–163
Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C (2008) Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 40:1044–1055
Mukherjee P, Miller JH, Shimony JS et al (2002) Diffusion-tensor MR imaging of gray and white matter development during of normal human brain maturation. AJNR AM J Neuroradiol 232:1445–1456
Zhang L, Thomas KM, Davidson MC et al (2005) MR quantitation volume and diffusion changes in the developing brain. AJNR Am J Neuroradiol 26:45–49
Hsu J-L, Leemans A, Bai C-H et al (2008) Gender differences and age-related white matter changes of the human brain: a diffusion tensor imaging study. Neuroimage 39:566–577
Acknowledgement
This study was supported in part by a Grant-in-aid from Japan Society for the Promotion of Science (no. 1859351).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshiura, T., Noguchi, T., Hiwatashi, A. et al. Intra- and interhemispheric variations of diffusivity in subcortical white matter in normal human brain. Eur Radiol 20, 227–233 (2010). https://doi.org/10.1007/s00330-009-1534-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-009-1534-z