Abstract
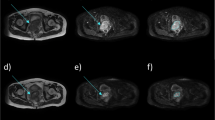
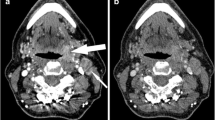
Our purpose was to determine the repeatability of squamous cell cancer in head and neck (SCCHN) and muscle tissue vascularity measurements as well as the inter- and intra-observer agreement using dynamic contrast-enhanced (DCE) multi-detector CT (MDCT). Twelve patients with histologically proven SCCHN were twice examined within 46 h. Measurement error and repeatability were assessed for each of the four functional parameters using the Bland-Altman plots. Two independent observers recorded the vascularity values of the tumor tissue; inter- and intra-observer agreement was assessed using the Bland-Altman plot analysis and intraclass correlation coefficients. For the tumor, the mean difference (95% limits of agreement) was 0.40 ml/min/100 g tissue (−6.80, 9.60); 0.01 (−0.96, 0.97) ml/100 g tissue; 0.20 (−1.80, 2.30) s; and 0.40 (−2.00, 2.80) ml/min/100 g tissue for BF, BV, MTT, and PS, respectively. For the muscle, the mean difference (95% limits of agreement) was −0.18 (−1.70, 1.35), 0.04 (−1.17, 1.35), −0.10 (−5.80, 5.60), and −0.10 (−2.20, 2.00), respectively. Measurement changes of at least ±8%, 30%, 36%, and 13% were found to be significant for BF, BV, MTT, and PS, respectively. There was better intra- than inter-observer agreement.


Similar content being viewed by others
References
Bisdas S, Baghi M, Smolarz A et al (2007) Quantitative measurements of perfusion and permeability of oropharyngeal and oral cavity cancer, recurrent disease, and associated lymph nodes using first-pass contrast-enhanced computed tomography studies. Invest Radiol 42:172–179
Gandhi D, Hoeffner EG, Carlos RC et al (2003) Computed tomography perfusion of squamous cell carcinoma of the upper aerodigestive tract. Initial results. J Comput Assist Tomogr 27:687–693
Rumboldt Z, Al-Okaili R, Deveikis JP (2005) Perfusion CT for head and neck tumors: pilot study. AJNR Am J Neuroradiol 26:1178–1185
Zima A, Carlos R, Gandhi D et al (2007) Can pretreatment CT perfusion predict response of advanced squamous cell carcinoma of the upper aerodigestive tract treated with induction chemotherapy? AJNR Am J Neuroradiol 28:328–334
Hermans R, Lambin P, Van der Goten A et al (1999) Tumoural perfusion as measured by dynamic computed tomography in head and neck carcinoma. Radiother Oncol 53:105–111
Cenic A, Nabavi DG, Craen RA et al (2000) A CT method to measure hemodynamics in brain tumors: validation and application of cerebral blood flow maps. AJNR Am J Neuroradiol 21:462–470
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Goh V, Halligan S, Hugill JA et al (2006) Quantitative assessment of tissue perfusion using MDCT: comparison of colorectal cancer and skeletal muscle measurement reproducibility. AJR 187:164–169
Ng QS, Goh V, Klotz E et al (2006) Quantitative assessment of lung cancer perfusion using MDCT: does measurement reproducibility improve with greater tumor volume coverage? AJR 187:1079–1084
Cenic A, Nabavi DG, Craen RA et al (1999) Dynamic CT measurement of cerebral blood flow: a validation study. AJNR Am J Neuroradiol 20:63–73
Nabavi DG, Cenic A, Dool J et al (1999) Quantitative assessment of cerebral hemodynamics using CT: stability, accuracy, and precision studies in dogs. J Comput Assist Tomogr 23:506–515
Goh V, Halligan S, Hugill JA et al (2005) Quantitative assessment of colorectal cancer perfusion using MDCT: inter- and intraobserver agreement. AJR 185:225–231
Fiorella D, Heiserman J, Prenger E et al (2004) Assessment of the reproducibility of postprocessing dynamic CT perfusion data. AJNR Am J Neuroradiol 25:97–107
Miles KA, Griffiths MR (2003) Perfusion CT: a worthwhile enhancement? Br J Radiol 76:220–231
Blomley MJ, Coulden R, Dawson P et al (1995) Liver perfusion studied with ultrafast CT. J Comput Assist Tomogr 19:424–433
Waaijer A, van der Schaaf IC, Velthuis BK et al (2007) Reproducibility of quantitative CT brain perfusion measurements in patients with symptomatic unilateral carotid artery stenosis. AJNR Am J Neuroradiol 28:927–932
Nabavi DG, Cenic A, Craen RA et al (1999) CT assessment of cerebral perfusion: experimental validation and initial clinical experience. Radiology 213:141–149
Erasmus JJ, Gladish GW, Broemeling L et al (2003) Interobserver and intraobserver variability in measurement of non-small-cell carcinoma lung lesions: implications for assessment of tumor response. J Clin Oncol 21:2574–2582
Vos MJ, Uitdehaag BM, Barkhof F et al (2003) Interobserver variability in the radiological assessment of response to chemotherapy in glioma. Neurology 60:826–830
Sanelli PC, Nicola G, Tsiouris AJ et al (2007) Reproducibility of postprocessing of quantitative CT perfusion maps. AJR 188:213–218
Sanelli PC, Nicola G, Johnson R et al (2007) Effect of training and experience on qualitative and quantitative CT perfusion data. AJNR Am J Neuroradiol 28:428–432
Leach MO, Brindle KM, Evelhoch JL et al (2005) The assessment of antiangiogenic and antivascular therapies in early-stage clinical trials using magnetic resonance imaging: issues and recommendations. Br J Cancer 92:1599–1610
Rudin M, McSheehy PM, Allegrini PR et al (2005) PTK787/ZK222584, a tyrosine kinase inhibitor of vascular endothelial growth factor receptor, reduces uptake of the contrast agent GdDOTA by murine orthotopic B16/BL6 melanoma tumours and inhibits their growth in vivo. NMR Biomed 18:308–321
Kan Z, Phongkitkarun S, Kobayashi S et al (2005) Functional CT for quantifying tumor perfusion in antiangiogenic therapy in a rat model. Radiology 237:151–158
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bisdas, S., Surlan-Popovic, K., Didanovic, V. et al. Functional CT of squamous cell carcinoma in the head and neck: repeatability of tumor and muscle quantitative measurements, inter- and intra-observer agreement. Eur Radiol 18, 2241–2250 (2008). https://doi.org/10.1007/s00330-008-0990-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-008-0990-1