Abstract
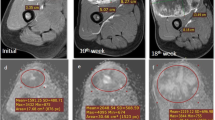
The objective of this study was to evaluate whether dynamic contrast-enhanced MR imaging can determine tumor response and localize residual viable tumor after isolated limb perfusion (ILP) chemotherapy in soft tissue tumors. Twelve consecutive patients, with histologically proven high-grade soft tissue sarcoma, prospectively underwent non-enhanced MR and dynamic contrast-enhanced MR imaging before and after ILP. Tumor volume was measured on non-enhanced MR images. The temporal change of signal intensity in a region of interest on dynamic contrast-enhanced MR images was plotted against time. Start, pattern, and progression of enhancement were recorded. Histopathologic response was defined as complete response if no residual viable tumor was present, partial remission if <50% viable tumor was present, and no change if ≥50% viable tumor was present in the resection specimen. Resected specimens for correlation with histopathology were available for 10 patients; 5 patients had partial remission and 5 had no change. Volume measurements correctly predicted tumor response in 6 of 10 patients. Dynamic contrast-enhanced MR correctly predicted tumor response in 8 of 10 patients. Early rapidly progressive enhancement correlated histologically with residual viable tumor. Late and gradual, or absence of enhancement, was associated with necrosis, predominantly centrally located, or granulation tissue. These preliminary results show that dynamic contrast-enhanced MR imaging offers potential for non-invasive monitoring of response to isolated limb perfusion in soft tissue sarcomas due to identification of residual areas of viable tumor and subsequently may provide clinically useful information with regards to timing and planning of additional surgery. Further prospective studies in a larger patient population is warranted.


Similar content being viewed by others
References
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285:1182–1186
Denekamp J (1993) Review article: angiogenesis, neovascular proliferation and vascular pathophysiology as targets for cancer therapy. Br J Radiol 66:181–196
Lienard D, Ewalenko P, Delmotte JJ, Renard N, Lejeune FJ (1992) High-dose recombinant tumor necrosis factor alpha in combination with interferon gamma and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J Clin Oncol 10:52–60
Eggermont AM, Schraffordt Koops H, Klausner JM et al. (1996) Isolated limb perfusion with tumor necrosis factor and melphalan for limb salvage in 186 patients with locally advanced soft tissue extremity sarcomas. The cumulative multicenter European experience. Ann Surg 224:756–764
Eggermont AM, Schraffordt Koops H, Lienard D et al. (1996) Isolated limb perfusion with high-dose tumor necrosis factor-alpha in combination with interferon-gamma and melphalan for nonresectable extremity soft tissue sarcomas: a multicenter trial. J Clin Oncol 14:2653–2665
Renard N, Lienard D, Lespagnard L, Eggermont AM, Heimann R, Lejeune F (1994) Early endothelium activation and polymorphonuclear cell invasion precede specific necrosis of human melanoma and sarcoma treated by intravascular high-dose tumour necrosis factor alpha (rTNF alpha). Int J Cancer 57:656–663
Issakov J, Merimsky O, Gutman M et al. (2000) Hyperthermic isolated limb perfusion with tumor necrosis factor-alpha and melphalan in advanced soft-tissue sarcomas: histopathological considerations. Ann Surg Oncol 7:155–159
Olieman AF, van Ginkel RJ, Hoekstra HJ, Mooyaart EL, Molenaar WM, Schraffordt Koops H (1997) Angiographic response of locally advanced soft-tissue sarcoma following hyperthermic isolated limb perfusion with tumor necrosis factor. Ann Surg Oncol 4:64–69
Carrasco CH, Charnsangavej C, Raymond AK et al. (1989) Osteosarcoma: angiographic assessment of response to preoperative chemotherapy. Radiology 170:839–842
Delorme S, Knopp MV (1998) Non-invasive vascular imaging: assessing tumour vascularity. Eur Radiol 8:517–527
Erlemann R, Sciuk J, Bosse A et al. (1990) Response of osteosarcoma and Ewing sarcoma to preoperative chemotherapy: assessment with dynamic and static MR imaging and skeletal scintigraphy. Radiology 175:791–796
Erlemann R (1993) Dynamic, gadolinium-enhanced MR imaging to monitor tumor response to chemotherapy. Radiology 186:904–905
Hanna SL, Parham DM, Fairclough DL, Meyer WH, Le AH, Fletcher BD (1992) Assessment of osteosarcoma response to preoperative chemotherapy using dynamic FLASH gadolinium-DTPA-enhanced magnetic resonance mapping. Invest Radiol 27:367–373
Reddick WE, Bhargava R, Taylor JS, Meyer WH, Fletcher BD (1995) Dynamic contrast-enhanced MR imaging evaluation of osteosarcoma response to neoadjuvant chemotherapy. J Magn Reson Imaging 5:689–694
van der Woude HJ, Bloem JL, Verstraete KL, Taminiau AH, Nooy MA, Hogendoorn PC (1995) Osteosarcoma and Ewing's sarcoma after neoadjuvant chemotherapy: value of dynamic MR imaging in detecting viable tumor before surgery. Am J Roentgenol 165:593–598
Shapeero LG, Henry-Amar M, Vanel D (1992) Response of osteosarcoma and Ewing sarcoma to preoperative chemotherapy: assessment with dynamic and static MR imaging and skeletal scintigraphy. Invest Radiol 27:989–991
Creech O Jr, Krementz E (1966) Techniques of regional perfusion. Surgery 60:938–947
Verstraete KL, Achten E, Dierick A (1992) Dynamic contrast-enhanced MRI of musculo-skeletal neoplasms: different types and slopes of time—intensity curves (abstr). In: Books of abstracts. Society of Magnetic Resonance in Medicine 1992. Society of Magnetic Resonance in Medicine, Berkeley, California
Clamon G, Clamon L (1993) Relationship between tumor area, tumor volume, and criteria of response in clinical trials. J Clin Oncol 11:1005
De Schepper AM, Parizel PM, De Beuckeleer L, Vanhoenacker F (2001) Imaging of soft tissue tumors, 2nd edn. Springer, Berlin Heidelberg New York
van der Woude HJ, Verstraete KL, Hogendoorn PC, Taminiau AH, Hermans J, Bloem JL (1998) Musculoskeletal tumors: Does fast dynamic contrast-enhanced subtraction MR imaging contribute to the characterization? Radiology 208:821–828
Verstraete KL, De Deene Y, Roels H, Dierick A, Uyttendaele D, Kunnen M (1994) Benign and malignant musculoskeletal lesions: dynamic contrast-enhanced MR imaging: parametric "first-pass" images depict tissue vascularization and perfusion. Radiology 192:835–843
Eroglu A, Kocaoglu H, Demirci S, Akgul H (2000) Isolated limb perfusion with cisplatin and doxorubicin for locally advanced soft tissue sarcoma of an extremity. Eur J Surg Oncol 26:213–221
Moertel CG, Hanley JA (1976) The effect of measuring error on the results of therapeutic trials in advanced cancer. Cancer 38:388–394
Fletcher BD, Hanna SL, Fairclough DL, Gronemeyer SA (1992) Pediatric musculoskeletal tumors: use of dynamic, contrast-enhanced MR imaging to monitor response to chemotherapy. Radiology 184:243–248
Schmidt RA, Conrad EU III, Collins C, Rabinovitch P, Finney A (1993) Measurement and prediction of the short-term response of soft tissue sarcomas to chemotherapy. Cancer 72:2593–2601
Panicek DM, Casper ES, Brennan MF, Hajdu SI, Heelan RT (1991) Hemorrhage simulating tumor growth in malignant fibrous histiocytoma at MR imaging. Radiology 181:398–400
Kettelhack C, Wickede M, Vogl T, Schneider U, Hohenberger P (2002) 31Phosphorus-magnetic resonance spectroscopy to assess histologic tumor response noninvasively after isolated limb perfusion for soft tissue tumors. Cancer 94:1557–1564
De Schepper AM, De Beuckeleer L, Vandevenne J, Somville J (2000) Magnetic resonance imaging of soft tissue tumors. Eur Radiol 10:213–223
Taylor JS, Tofts PS, Port R et al. (1999) MR imaging of tumor microcirculation: promise for the new millennium. J Magn Reson Imaging 10:903–907
Lang P, Vahlensieck M, Matthay KK et al. (1996) Monitoring neovascularity as an indicator to response to chemotherapy in osteogenic and Ewing sarcoma using magnetic resonance angiography. Med Pediatr Oncol 26:329–333
Shapeero LG, Vanel D, Verstraete KL, Bloem JL (1999) Dynamic contrast-enhanced MR imaging for soft tissue sarcomas. Semin Musculoskelet Radiol 3:101–114
Shapeero LG, Vanel D, Verstraete KL, Bloem JL (2002) Fast magnetic resonance imaging with contrast for soft tissue sarcoma viability. Clin Orthop 2002:212–227
Jones DN, McCowage GB, Sostman HD et al. (1996) Monitoring of neoadjuvant therapy response of soft-tissue and musculoskeletal sarcoma using fluorine-18-FDG PET. J Nucl Med 37:1438–1444
van Ginkel RJ, Hoekstra HJ, Pruim J et al. (1996) FDG-PET to evaluate response to hyperthermic isolated limb perfusion for locally advanced soft-tissue sarcoma. J Nucl Med 37:984–990
van Ginkel RJ, Kole AC, Nieweg OE et al. (1999) L-[1–11C]-tyrosine PET to evaluate response to hyperthermic isolated limb perfusion for locally advanced soft-tissue sarcoma and skin cancer. J Nucl Med 40:262–267
Sijens PE, Eggermont AM, van Dijk PV, Oudkerk M (1995) 31P magnetic resonance spectroscopy as predictor of clinical response in human extremity sarcomas treated by single dose TNF-alpha+melphalan isolated limb perfusion. NMR Biomed 8:215–224
Redmond OM, Bell E, Stack JP et al. (1992) Tissue characterization and assessment of preoperative chemotherapeutic response in musculoskeletal tumors by in vivo 31P magnetic resonance spectroscopy. Magn Reson Med 27:226–237
Brasch RC, Li KC, Husband JE et al. (2000) In vivo monitoring of tumor angiogenesis with MR imaging. Acad Radiol 7:812–823
Egmont-Petersen M, Hogendoorn PC, van der Geest RJ et al. (2000) Detection of areas with viable remnant tumor in postchemotherapy patients with Ewing's sarcoma by dynamic contrast-enhanced MRI using pharmacokinetic modeling. Magn Reson Imaging 18:525–535
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Rijswijk, C.S.P., Geirnaerdt, M.J.A., Hogendoorn, P.C.W. et al. Dynamic contrast-enhanced MR imaging in monitoring response to isolated limb perfusion in high-grade soft tissue sarcoma: initial results. Eur Radiol 13, 1849–1858 (2003). https://doi.org/10.1007/s00330-002-1785-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-002-1785-4