Abstract
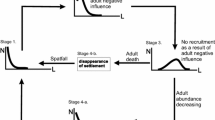
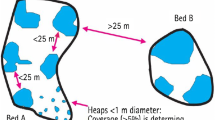
Mussel beds are common in many intertidal and subtidal habitats, including sub-Arctic and Arctic regions. Despite their ecological importance, little is known about the demographic processes governing the development of mussel beds and particularly about the synchronization of these processes at different spatial scales. The development of a relatively compact, narrow littoral mussel bed of Mytilus trossulus, extending several hundred m along the shore of Koni Peninsula (Tauyskaya Bay, northern Sea of Okhotsk), was studied for four years during the period 2014–2017. This model bed was located along an exposed coast with a seasonal sea ice cover. It was used to test the hypothesis that small-scale synchronization exists, i.e., the changes in demographic structure of the population that occur simultaneously within a single mussel bed follow a similar pattern. This hypothesis was rejected. The size structure of the mussel population at the same tidal level changed from being uniform (2014) to being varied in 2015–2017. The course of development of the mussel population was different at the opposite ends of the bed. The cause of these differences may be unequal levels of recruitment of juveniles in the form of a gradient from one end of the bed to the other over the length of the bed. The gradient was associated with transition from one type of shore dynamic processes to another (accumulation vs. abrasion). Small-scale desynchronization may represent one of the reasons explaining the heterogeneity of mussel populations. The possibility of desynchronization of demographic processes should be taken into consideration when studying the spatial organization and dynamics of mussel beds.




Similar content being viewed by others
References
Airoldi L (2003) The effects of sedimentation on rocky coast assemblages. Oceanogr Mar Biol 41:161–236
Alvarado JL, Castilla JC (1996) Tridimensional matrices of mussels Perumytilus purpuratus on intertidal platforms with varying wave forces in central Chile. Mar Ecol Prog Ser 133:135–141
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Anderson MJ (2005) Permutational multivariate analysis of variance. Department of Statistics, University of Auckland, Auckland
Antsulevich AE, Maximovich NV, Vuorinen I (1999) Population structure, growth and reproduction of the common mussel (Mytilus edulis L.) off the Island of Seili (SW Finland). Boreal Env Res 4:368–375
Bayne BL (1976) Marine mussels: their ecology and physiology. International Biological Programme, 10. Cambridge University Press, Cambridge
Beukema JJ, Dekker R, Essink K, Michaelis H (2001) Synchronized reproductive success of the main bivalve species in the Wadden Sea: causes and consequences. Mar Ecol Prog Ser 211:143–155
Beukema JJ, Dekker R (2007) Variability in annual recruitment success as a determinant of long-term and large-scale variation in annual production of intertidal Wadden Sea mussels (Mytilus edulis). Helgol Mar Res 61:71–86
Beukema JJ, Dekker R, van Stralen MR, de Vlas J (2015) Large-scale synchronization of annual recruitment success and stock size in Wadden Sea populations of the mussel Mytilus edulis L. Helgol Mar Res 69:327–333
Blicher ME, Sejr MK, Høgslund S (2013) Population structure of Mytilus edulis in the intertidal zone in a sub-Arctic fjord, SW Greenland. Mar Ecol Prog Ser 487:89–100
Buschbaum C, Saier B (2001) Growth of the mussel Mytilus edulis L. in the Wadden Sea affected by tidal emergence and barnacle epibionts. J Sea Res 45:27–36
Buschbaum C, Dittmann S, Hong J-S, Hwang I-S, Strasser M, Thiel M, Valdivia N, Yoon S-P, Reise K (2009) Mytilid mussels: global habitat engineers in coastal sediments. Helgol Mar Res 63:47–58
Buyanovski AI (1996) Population structure and dynamics of mussels Mytilus trossulus (Bvalvia, Mytilidae) in the foulings of Avacha Bay (Eastern Kamchatka). Zoologicheskii Zhurnal 75:28–34 (in Russian)
Casagrandi R, Mari L, Gatto M (2007) Modelling the local dynamics of the zebra mussel (Dreissena polymorpha). Freshw Biol 52:1223–1238
Chernyavski VI, YaG R (1994) Physical and geographical characteristics of the Tauisk Bay of the Sea of Okhotsk. Proc GosNIORH 308:10–24 (in Russian)
Commito JA, Commito AE, Platt RV, Grupe BM, Dow Piniak WE, Gownaris NJ, Reeves KA, Vissichelli AM (2014) Recruitment facilitation and spatial pattern formation in soft-bottom mussel beds. Ecosphere 5(12):160
Commito JA, Dow WE, Grupe BM (2006) Hierarchical spatial structure in soft-bottom mussel beds. J Exp Mar Biol Ecol 330:27–37
Cusson M, Bourget E (2005) Small-scale variations in mussel (Mytilus spp.) dynamics and local production. J Sea Res 53:255–268
Derkacheva AA (2014) Types of the shores of Koni Peninsula Chronicle of Nature of the Magadan State. Nat Reserve For 1:13–22
Dolmer P, Stenalt E (2010) The impact of the adult blue mussel (Mytilus edulis) population on settling of conspecific larvae. Aquacult Int 18:3–17
Essink K, Bos AH (1985) Growth of three bivalve molluscs transplanted along the axis of the Ems estuary. Neth J Sea Res 19:45–51
Filippov AA (2007) Adaptive capacities of the mussel Mytilus edulis (Bivalvia, Mytilidae) for changes in salinity of the White Sea water. Zoologicheskii zhurnal 86:415–420 (in Russian)
Folmer EO, Drent J, Troost K, Büttger H, Dankers N, Jansen J, van Stralen M, Millat G, Herlyn M, Philippart CJM (2014) Large-scale spatial dynamics of intertidal mussel (Mytilus edulis L.) bed coverage in the German and Dutch Wadden Sea. Ecosystems 17:550–566
Gerasimova AV, Maximovich NV (2013) Age–size structure of common bivalve mollusc populations in the White Sea: the causes of instability. Hydrobiologia 706:119–137
Griffiths RJ (1981) Population dynamics and growth of the bivalve Choromytilus meridionalis (Rr.) at different tidal levels. Estuar Coast Shelf Sci 12:101–118
Griffiths CL, Hockey PAR (1987) A model describing the interactive roles of predation, competition and tidal elevation in structuring mussel population. S Afr J Mar Sci 5:547–556
Hunt HL, Scheibling RE (1996) Physical and biological factors influencing mussel (Mytilus trossulus, M. edulis) settlement on a wave-exposed rocky shore. Mar Ecol Prog Ser 142:135–145
Hutchison ZL, Hendrick VJ, Burrows MT, Wilson B, Last KS (2016) Buried alive: the behavioural response of the mussels, Modiolus modiolus and Mytilus edulis to sudden burial by sediment. PLoS ONE 11(3):e0151471. https://doi.org/10.1371/journal.pone.0151471
Kautsky N (1982) Growth and size structure in a Baltic Mytilus edulis population. Mar Biol 68:117–133
Khaitov VM (2013) Life in an unstable house: community dynamics in changing mussel beds. Hydrobiologia 706:139–158
Khaitov VM, Lentsman NV (2016) The cycle of mussels: long-term dynamics of mussel beds on intertidal soft bottoms at the White Sea. Hydrobiologia 781:161–180
Khalaman VV, Flyachinskaya LP, Lezin PA (2009) Impact of excretoty-secretory products of some fouling organisms on settling of mussel’s larvae (Mytilus edulis L., Bivalvia, Mollusca). Invertebr Zool 6:65–72 (in Russian)
Kostylev V, Erlandsson J (2001) A fractal approach for detecting spatial hierarchy and structure on mussel beds. Mar Biol 139:497–506
Kulakovski EE, Shamarin AYu (1989) The peculiarities of young mussel (Mytilus edulis L.) settlement and growth in the experimental-industrial aquaculture in the White Sea. Proc Zool Inst USSR AS 203:63–75 (in Russian)
Lawrie SM, McQuaid CD (2001) Scales of mussel bed complexity: structure, associated biota and recruitment. J Exp Mar Biol Ecol 257:135–161
Lehane C, Davenport J (2004) Ingestion of bivalve larvae by Mytilus edulis: experimental and field demonstrations of larviphagy in farmed blue mussels. Mar Biol 145:101–107
Leonard GH, Levine JM, Schmidt PR, Bertness MD (1998) Flow-driven variation in intertidal community structure in a Maine Estuary. Ecology 79:1395–1411
Lisitsyn AP (1995) The marginal filter of the ocean. Oceanology 34:671–682
Lukanin VV, Naumov AD, Fedyakov VV (1986) The cyclic development of Mytilus edulis L. populations in the White Sea. Doklady Akademii Nauk SSSR 287:78–84 (in Russian)
Lukanin VV, Oshurkov VV (1981) Structure of littoral populations of mussel in Kandalaksha Bay of the White Sea. Russ J Mar Biol 5:33–38 (in Russian)
Maximovich NV, Sukhotin AA, Minichev YuS (1996) Long-term dynamics of blue mussel (Mytilus edulis L.) culture settlements (the White Sea). Aquaculture 147:191–204
McGrorty S, Clarke RT, Reading CJ, Goss-Custard JD (1990) Population dynamics of the mussel Mytilus edulis: density changes and regulation of the population in the Exe estuary, Devon. Mar Ecol Prog Ser 67:157–169
McQuaid CD, Lindsay JR (2000) Effect of wave exposure on growth and mortality rates of the mussel Perna perna: bottom-up regulation of intertidal populations. Mar Ecol Prog Ser 206:147–154
McQuaid CD, Lindsay JR (2005) Interacting effects of wave exposure, tidal height and substratum on spatial variation in densities of mussel Perna perna plantigrades. Mar Ecol Prog Ser 301:173–184
McQuaid CD, Mostert BP (2010) The effects of within-shore water movement on growth of the intertidal mussel Perna perna: an experimental field test of bottom-up control at centimetre scales. J Exp Mar Biol Ecol 384:119–123
Menge BA (1992) Community regulation: under what conditions are bottom-up factors important on rocky shores? Ecology 73:755–765
Naumov AD (2006) Clams of the White sea: ecological and faunistic analysis. Zoological Institute RAS, Saint-Petersburg (in Russian)
Naumov AD (2013) Long-term fluctuations of soft-bottom intertidal community structure affected by ice cover at two small sea bights in the Chupa Inlet (Kandalaksha Bay) of the White Sea. Hydrobiologia 706:159–173
Nehls G, Thiel M (1993) Large-scale distribution patterns of the mussel Mytilus edulis in the Wadden Sea of Schleswig-Holstein: do storms structure the ecosystems? Neth J Sea Res 31:181–187
Öst M, Kilpi M (1997) A recent change in size distribution of blue mussels (Mytilus edulis) in the western part of the Gulf of Finland. Ann Zoologici Fennici 34:31–36
Peharda M, Ezgeta-Balić D, Davenport J, Bojanić N, Vidjak O, Ninčević-Gladan Ž (2012) Differential ingestion of zooplankton by four species of bivalves (Mollusca) in the Mali Ston Bay, Croatia. Mar Biol 159:881–895
Porri F, Jordaan T, McQuaid C (2008) Does cannibalism of larvae by adults affect settlement and connectivity of mussel populations? Estuar Coast Shelf Sci 79:687–693
Remane A (1971) Ecology of brackish water. In: Remane A, Schlieper C (eds) Biology of brackish water. Wiley Interscience, New York, pp 1–210
Riisgård HU, Larsen PS, Turja R, Lundgreen K (2014) Dwarfism of blue mussels in the low saline Baltic Sea—growth to the lower salinity limit. Mar Ecol Prog Ser 517:181–192
Smaal AC (2002) European mussel cultivation along the Atlantic coast: production status, problems and perspectives. Hydrobiologia 484:89–98
Spiridonov VA, Neretin NY, Spiridonov VA, Anosov SE (2013) Studies of the littoral of the Magadan State Nature Reserve. Report on the grant provided by UNDP/GEF Project “Strengthening of marine protected areas in Russia” to the Magadan State Nature Reserve (in Russian)
Steffani CN, Branch GM (2003) Growth rate, condition and shell shape of Mytilus galloprovincialis: responses to wave exposure. Mar Ecol Prog Ser 246:197–209
Strasser M, Reinwald T, Reise K (2001) Differential effects of the severe winter of 1995/96 on the intertidal bivalves Mytilus edulis, Cerastoderma edule and Mya arenaria in the Northern Wadden Sea. Helg Mar Res 55:190–197
Sukhotin AA, Kulakowski EE (1992) Growth and population dynamics in mussels (Mytilus edulis L.) cultured in the White Sea. Aquaculture 101:59–73
Svane I, Ompi M (1993) Patch dynamics in beds of the blue mussel Mytilus edulis L.: effects of site, patch size, and position within patch. Ophelia 37:187–202
Torroglosa ME, Giménez J (2019) Responses of mussel Brachidontes rodriguezii (d´Orbigny, 1842) to aerial exposure: implications on growth and physiological condition. Can J Zool 97:612–618
Vuorinen I, Antsulevich AE, Maximovich NV (2002) Spatial distribution and growth of the common mussel Mytilus edulis L. in the archipelago of SW-Finland, northern Baltic Sea. Boreal Environ Res 28:41–52
Westerbom M, Jattu S (2006) Effects of wave exposure on the sublittoral distribution of blue mussels Mytilus edulis in a heterogeneous archipelago. Mar Ecol Prog Ser 306:191–200
Westerbom M, Mustonen O, Kilpi M (2008) Distribution of a marginal population of Mytilus edulis: responses to biotic and abiotic processes at different spatial scales. Mar Biol 153:1153–1164
Yaroslavtseva LM, Sergeeva EP (2006) Adaptivity of the Bivalve Mytilus trossulus larvae to short- and long-term changes in water temperature and salinity. Russ J Mar Biol 32:82–87
Zharnikov VS (2011) The Mytilus trossulus (Bivalvia: Mytilidae) larvae in the meroplankton of Tauiskaya Bay, the Sea of Okhotsk. Bull North-East Sci Center RAS, Far East Branch 4:101–104 (in Russian)
Zharnikov VS (2014) Number dynamics of the mussel Mytilus trossulus (Bivalvia: Mytilidae) larvae and their settlement on artificial substrata that were located in water column and in littoral zone of the Vesyolaya Bay, Tauy Inlet, Sea of Okhotsk. Bull North-East Sci Center RAS, Far East Branch 1:55–62 (in Russian)
Acknowledgements
We would like to thank Alice Lagnado for editing the English language of our manuscript. The authors also express profound gratitude to the administration (director Yury Berezhnoi, deputy directors Irina Utekhina and Andrei Kotyukh) and staff of the Magadan Nature Reserve for providing the opportunity to conduct this study, and their constant and invaluable assistance with the field work. This work was supported by the Russian Foundation for Basic Research [Grant Numbers 14-04-10159, 13-04-01127]; Program of the field studies for bachelor and master studies of the Perm National Research University in 2014 and 2016; Basic research funds of the Magadan State Nature Reserve; and State Task [Reg. Number AAAA-A19-119022690122-5].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare the absence of any conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khalaman, V.V., Trunova, A.D., Tridrikh, N.N. et al. From uniformity to multiplicity: development of a sub-arctic Littoral Mussel Bed in the Sea of Okhotsk. Polar Biol 43, 1341–1352 (2020). https://doi.org/10.1007/s00300-020-02712-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-020-02712-4