Abstract
Global warming is causing Atlantification of water masses and concomitant changes in food webs in the Barents Sea region. To determine whether changes that have been documented at lower trophic levels are impacting the diet of ringed seals (Pusa hispida) gastrointestinal tracts (GITs) from 99 coastal-feeding ringed seals, collected in western Spitsbergen, Svalbard, were analysed via identification of hard-parts. The study animals were shot in spring (n = 30; April–July) or autumn (n = 69; August–October) during four consecutive years (2014–2017). Thirty different prey types were identified, but most seals (55.6%) had consumed between 2 and 4 different types of prey. Polar cod (Boreogadus saida) dominated the diet of the ringed seals in terms of relative biomass (Bi = 60.0%) and frequency of occurrence (FOi = 86.9%), followed by pricklebacks (Stichaeidae; Bi = 23.4%; FOi = 79.8%). Redundancy analysis (RDA) revealed that year was the only significant predictor explaining variance in autumn diet composition (RDA, F3 = 4.96, AIC = − 76.49, p ≤ 0.0050; blubber content and maturity/sex group were not significant). Blue whiting (Micromesistius poutassou) occurred in the diet in small quantities; this Atlantic fish species has not previously been documented in the ringed seals’ diet. Atlantic cod (Gadus morhua) had the highest Bi (9.2%) among Atlantic prey types. However, despite major changes in the last decade in the fish and zooplankton community in western Svalbard, and consumption of a few Atlantic prey types, the ringed seals’ diet in Svalbard continues to be dominated by Arctic prey, especially polar cod.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ringed seal (Pusa hispida hispida) is an important species in Arctic food webs, both as a predator of a variety of fish and invertebrate species (McLaren 1958; Labansen et al. 2007; Crawford et al. 2015) and as a primary prey species for polar bears (Ursus maritimus; Stirling and Øritsland 1995; Iversen et al. 2013) and coastal people in the Arctic (Teilmann and Kapel 1998). In addition, it is food for a wide variety of other species, such as Greenland sharks (Somniosus microcephalus), walruses (Odobenus rosmarus), Arctic foxes (Vulpes lagopus), glaucous gulls (Larus hyperboreus) and killer whales (Orcinus orca) (Smith 1976; Lowry and Fay 1984; Lydersen and Smith 1989; Melnikov and Zagrebin 2005; Leclerc et al. 2012). The ringed seal is a circumpolar species and is thought to be one of the most abundant seal species in the Arctic (Reeves 1998). Although few abundance estimates exist, and even fewer time series of population trends, there is concern for the status of ringed seals with regard to global warming and concomitant sea ice declines (e.g. Laidre et al. 2015). Local declines have been reported in some areas for ringed seals (e.g. Ferguson et al. 2017) and are suspected in other regions (Hamilton et al. 2019a).
Ringed seals give birth in snow lairs on either land fast ice or drifting pack ice (McLaren 1958; Finley et al. 1983; Wiig et al. 1999). In the Svalbard Archipelago, ringed seals are generally born in early April. Lactation lasts for about 39 days (Hammill et al. 1991), after which females mate. During the reproductive season, adult males actively defend underwater territories that encompass the lair complexes used by several females (Ryg and Øritsland 1991; Lydersen 1998). Moulting takes place post-breeding, usually starting in late May and lasting for a period of approximately one month, though moulting can extend through until the end of July (Ryg et al. 1990a; Gjertz et al. 2000; Freitas et al. 2008). Both the breeding season (including lactation and territorial defence) and the moulting period are energetically costly. Sexually mature ringed seals are generally in negative energy balance from April to July, losing a substantial amount of their stored blubber during this period (Ryg et al. 1990a; Hammill et al. 1991; Ryg and Øritsland 1991; Smith et al. 1991), despite some feeding during this time (Lydersen and Kovacs 1999). After moulting, Svalbard ringed seals remain associated with ice, travelling offshore, to areas along the ice edge (Freitas et al. 2008; Hamilton et al. 2015; Lone et al. 2019), or remaining in the fjords, where they use glacier ice as resting platforms and feed on concentrations of prey at up-welling areas at the front of tidewater glaciers (Hartley and Fisher 1936; Freitas et al. 2008; Lydersen et al. 2014; Hamilton et al. 2016, 2019b).
Arctic sea ice has decreased dramatically in recent decades and predictions for the future suggest that this trend will continue (Wang and Overland 2009; Overland and Wang 2010; IPCC 2014; Bilt et al. 2019). This raises concern for ringed seal populations throughout the Arctic (Tynan and DeMaster 1997; ACIA 2005; Simmonds and Isaac 2007; Laidre et al. 2008, 2015; Kovacs et al. 2011; Hamilton et al. 2015). Sea ice in the Svalbard area has declined profoundly, particularly on the west coast of the archipelago (Laidre et al. 2015; Lind et al. 2018). The North Atlantic Current (NAC) brings warm, saline Atlantic Water (AW) from the Gulf Stream into the Arctic Ocean via the Barents Sea. One of the main currents carrying this water northward is the West Spitsbergen Current (WSC), which runs along the coastal shelf slope, west of Spitsbergen (Tverberg et al. 2014). AW from the NAC has recently warmed markedly; AW was warmer at the beginning of this century than it has been during the last 2000 years (Spielhagen et al. 2011). Both the warmer temperatures of the WSC and increased inflow of this water into the fjords on the west coast of Spitsbergen, because of changing and more intense winds, has resulted in reduced sea ice formation (Cottier et al. 2005; Tverberg et al. 2014). A temperature peak in AW in the WSC was recorded in 2006, resulting in a large decrease in sea ice coverage in the region (Beszczynska-Möller et al. 2012; Lind and Ingvaldsen 2012), which has continued through to the present (Pavlova et al. 2019). In addition, the glaciers in Svalbard are experiencing a net-loss of mass due to the warmer climate (Nuth et al. 2010). This loss of mass is greatest for tidewater glaciers (Błaszczyk et al. 2009; Nuth et al. 2013) and many of these glaciers, whose fronts meet the ocean, are retreating onto land (Lindbäck et al. 2018; Bilt et al. 2019). Thus, ringed seals are likely to lose this important feeding and resting habitat in the future in Svalbard (Hamilton et al. 2016) and elsewhere.
The warming that has taken place has led to increases in the presence of Atlantic species in the marine food web in Svalbard (Søreide et al. 2013; Fossheim et al. 2015; Kortsch et al. 2015; Misund et al. 2016). Atlantic species such as Atlantic cod (Gadus morhua) and capelin (Mallotus villosus) are expanding their distribution northward (Drinkwater 2005; Hop and Gjøsæter 2013). Shallow water communities in Kongsfjorden are now dominated by Atlantic cod and shorthorn sculpin (Myoxocephalus scorpius), while polar cod (Boreogadus saida) have become rare (Brand and Fischer 2016). Atlantic mackerel (Scomber scombrus) (feeding on Atlantic herring (Clupea harengus)) were caught for the first time in Isfjorden on western Spitsbergen in 2013 (Berge et al. 2015). Potential consequences of these changes for ringed seals are unknown. However, there is concern that replacing lipid-rich Arctic prey species, e.g. polar cod and pricklebacks (Stichaeidae; Elliot and Gaston 2008) with less lipid-rich Atlantic prey species, e.g. Atlantic cod (Lawson et al. 1998), will be negative for these seals and other Arctic top predators. Although, this concern has been questioned because some Atlantic prey, e.g. Atlantic herring capelin and krill (Thysanoessa spp.) have high energy contents (Lawson et al. 1998; Elliot and Gaston 2008; Renaud et al. 2018).
Changes have been documented in ringed seal behaviour concomitant with the ice changes over recent decades that suggest that prey densities and sympagic availability of prey have declined. Ringed seals that travel to the ice edge north of Svalbard must travel longer distances to reach the ice and when they get into ice-covered areas they dive more, rest less and exhibit less area-restricted search in these areas, suggesting that they must search more broadly and that they encounter less concentrated prey schools (Hamilton et al. 2015). Additionally, they dive less frequently to just beneath the ice, suggesting that less sympagic prey is available now compared to a decade ago (Hamilton et al. 2015). Coastal ringed seals have retracted into glacier front habitats, and exhibit much smaller home ranges than previously following the unusually warm and ice-free year in 2006 (Hamilton et al. 2016), which seems to mark a biological turning point in this region, dictated by sea ice changes (see Vihtakari et al. 2018; Pavlova et al. 2019).
There is temporal and geographic variation in the diet of ringed seals (Lowry et al. 1980; Siegstad et al. 1998; Thiemann et al. 2007). On the west coast of Spitsbergen, Svalbard, previous studies have shown that the ringed seal diet was dominated by polar cod, with varying amounts of other fish species, such as pricklebacks, sculpins (Cottidae) and sebastids, in addition to a variety of invertebrate species e.g. Themisto libellula, Pandalus borealis, Gammarus wilkitzkii and krill (Gjertz and Lydersen 1986; Lydersen et al. 1989; Węsławski et al. 1994; Labansen et al. 2007). A recent stable isotope analysis conducted on ringed seal whiskers, collected in 1990 and 2013, suggested that their dietary has changed concomitant with ecosystem changes during the last decades (Lowther et al. 2017). However, whether this change is due to an altered diet of ringed seals or alternatively changes in the diet of their prey, cannot be distinguished through this method. The purpose of the present study was therefore to (1) study the diet of ringed seals, directly, via analyses of gastrointestinal tracts; (2) explore whether the diet of this important Arctic species has been impacted by the food web changes that have taken place in Svalbard over the past decade, by comparing results to previous dietary studies in the area; and (3) attempt to identify what factors might drive potential variance in diet composition of ringed seals in Svalbard.
Materials and methods
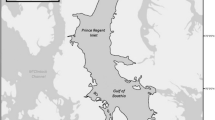
Gastrointestinal tracts (GITs) from 99 ringed seals were collected from animals shot by local sport hunters, from April to October during the years 2014–2017, at six locations in Isfjorden (Adolfbukta, Borebukta, Ekmanfjorden, Tempelfjorden, Yoldiabukta and Ymerbukta), and during April and May in 2014 in Van Mijenfjorden, on the west coast of Spitsbergen (Fig. 1). Hunters held “big game” licenses, which are mandatory for participation in sport-hunting seals in Svalbard. In the field, animals were weighed to the nearest 0.5 kg using a Salter 100-kg spring scale and standard body length was measured in a straight line from the nose to the tail (to the nearest cm; Scheffer 1967). Blubber thickness was measured dorsally at a position about 60% of the body length from behind the snout towards the tail, where blubber thickness is most variable (to the nearest mm; Ryg et al. 1988); all sampled animals appeared to be healthy. GITs were removed from the seal carcasses and tied shut at the oesophagus and the rectum before being frozen at − 20 °C until analysis. Reproductive organs and canine teeth (as well as various other tissues—for other studies) were collected and stored frozen at − 20 °C. In the laboratory, maturity of males was determined by the size of the testes (Ryg et al. 1991). Females were considered mature if a corpus luteum, c. albicans or a foetus was observed (McLaren and Smith 1985). Age was determined by counting cementum layers of decalcified and stained longitudinal sections of canines from the lower jaw (Lydersen and Gjertz 1987).
Stomachs, small intestines and large intestines were treated separately when their contents were handled in the lab, but sections were subsequently combined for most analyses to keep the individual animal as the sampling focus. After thawing, GIT sections were cut open and their contents were poured over a series of three connected sieves with mesh sizes of 2 mm, 1 mm and 0.5 mm (top to bottom). Otoliths and other prey materials that stuck to the containment bowl were collected directly. The contents on the sieve system were washed carefully with cold water and otoliths and invertebrate hard-part remains were collected. All collected material was preserved in 96% ethanol and subsequently examined under a Leica MZ6 stereomicroscope with an ocular micrometre. Sagittal fish otoliths (hereafter otoliths) and crustacean parts were identified to the lowest possible taxon with the help of the identification guides by Enckell (1980) and Härkönen (1986) and a reference collection of otoliths from fish caught on surveys around Svalbard and in the Barents Sea, provided by the Institute of Marine Research (IMR), Tromsø, Norway. Otoliths with minimal signs of erosion (i.e. surface structures clearly visible and not “smoothed”; e.g. Bowen and Harrison 1994) found in the stomach and small intestine were measured along the longest axis parallel to the sulcus (Härkönen 1986). Due to digestive erosion, otoliths from the large intestine were not measured. Fish length and mass, on a species-specific basis, were back-calculated using otolith length (Härkönen 1986; Windsland et al. 2007). When possible, otoliths from one species in a given part of the GIT were sorted into left and right and paired based on length before being measured, using the mean otolith lengths to calculate fish length and mass. A subsample of ~ 100 otoliths was measured when the number of otoliths from one species was > 100 in a given part of the GIT, assuming that the size distribution was representative for all otoliths in the sample. Otoliths in subsamples were not sorted into left and right. When estimating total biomass for each prey type, eroded and damaged otoliths were assumed to have the same overall size distribution as the measured otoliths from the same species in the same GIT. The number of otoliths found for each species in each GIT was divided by two (and then rounded up to a whole number—assuming both otoliths were ingested for each fish). For species in which otoliths can be sorted into left and right, the side with the highest count was used to estimate the number of fish consumed. Biomasses of various crustaceans were estimated by multiplying the number of individuals of a given species found in the GITs with average masses of that species caught in trawls around Svalbard (IMR, unpublished data).
Frequency of occurrence (FO), relative numerical frequency (Ni) and the relative proportion of biomass (Bi) of each prey item were used as diet indices (Hyslop 1980; Pierce and Boyle 1991) and calculated using following formulas: (1) \({FO}_{i}\left(\%\right)=\left(\frac{{S}_{i}}{{S}_{t}}\right)*100\), Si being the number of seals that had consumed prey type I and St the total number of seals; (2) \({N}_{i}\left(\%\right)=\left(\frac{{n}_{i}}{{n}_{t}}\right)*100\), where ni is the number prey type i consumed by all seals and nt the total number of prey consumed by all seals; (3) \({B}_{i}(\%)=\left(\frac{{b}_{i}}{{b}_{t}}\right)*100\), bi being the total biomass of prey type i and bt the total biomass of all estimated prey.
Percent blubber content of the seals (\(C(\%))\) was used as an indicator of body condition. This variable was calculated using (4) \( C\left( \% \right) = 5102*\sqrt {\frac{L}{M}} *d + 8.53 \), where \(L\) is body length in metres, \(M\) body mass in kilograms and \(d\) dorsal blubber thickness in metres (Ryg et al. 1990b). Year class (YC) of polar cod was estimated based on estimated fish length (back-calculated from otolith length) based on Falk-Petersen et al. (1986). Length intervals (mm) for the various age classes were: YC 1 ≤ 110.5; 110.5 < YC 2 ≤ 139.5; 139.5 < YC 3 ≤ 156.6; 156.6 < YC 4 ≤ 169.0; 169.0 < YC 5 ≤ 185.5; and YC 6 > 185.5.
Samples (stomachs, small intestines and large intestines) belonging the same GITs were pooled to represent the diet of individual seals. When analysing potential seasonal differences in ringed seal diet, samples collected in the period 04 April to 15 July, were grouped into a “spring-sample” (breeding and moulting periods, when food consumption is low) and samples collected in the period from 29 August to 10 October were grouped into an “autumn-sample” (active feeding/fattening season for ringed seals). This simplistic two-season division was performed because the total sample size of GITs in this study was small. To investigate whether prey consumed by the ringed seals were of Arctic or Atlantic origin, prey species belonging to families that are known to have year-round residency in Svalbard were classified as being Arctic—namely, polar cod, pricklebacks, eelpouts (Zoarcidae), sculpins, snailfish (Liparidae), Themisto libellula and Gammarus wilkitzkii. Due to the inability to identify all otoliths to species level, this assumes that only Arctic species were present for some families. The most common species in Svalbard waters belonging to these families are: pricklebacks, Lumpenus lampretaeformis, L. fabricii and Leptoclinus maculatus; eelpouts, Lycodes vahli, L. frigidus and Zoarces viviparus; sculpins, shorthorn sculpin and fourhorn sculpin (Myoxocephalus quadricornis); snailfish, Liparis liparis, L. fabricii and Careproctus reinhardti (Pethon 2005; Eriksen et al. 2012). The rest of the prey species found in the GITs were classified as Atlantic species.
Potential maturity/sex class (adult males, adult females and juveniles of both sexes) differences in diet of the ringed seals were explored using Chi-squared tests. To prevent expected values below five for some categories, due to low sample sizes, seals that had consumed between 0–2 and 5–11 different prey types were pooled into their respective maturity/sex groups before being tested. The distribution of the data sets on polar cod length and blubber thickness of seals were tested for normality using Shapiro–Wilk tests prior to analyses. Equal variances between samples were tested with F-tests (variances of two samples), Bartlett’s tests (variances of multiple samples with normal distributions) and Fligner-Killeen tests (variances of multiple samples with non-normal distributions). Differences between maturity/sex groups with regard to the lengths of polar cod ingested were tested with a Kruskal–Wallis rank sum test followed by pairwise Wilcoxon rank sum tests with Bonferroni corrections. To better understand what factors (biotic and abiotic) drive the variation in diet composition, a constrained ordination analysis (Legendre and Legendre 1998) was conducted on prey biomass data. Because there was a linear relationship (gradient length < 3) between the response matrix (diet matrix) variables and the predictor matrix, a redundancy analysis (RDA) was used for further analyses (Legendre and Anderson 1999; Corfield 2000; Lepš and Šmilauer 2003). The biomass of the five most important prey types (polar cod, pricklebacks, Atlantic cod, sculpins and krill were used as response variables and year, percent blubber content and maturity/sex group of the seals were used as predictor variables. Year and maturity/sex group were defined as nominal variables. To normalise the data and dampen the effect of outliers, the response variables (diet data) were transformed prior to the analysis using the Hellinger transformation (Legendre and Gallagher 2001; various transformations were explored). The RDA with untransformed data is presented in Online Resource 1. Model selection was done by testing predictor variables through forward selection using 1000 Monte Carlo permutations and ranking models by Akaike information criterion (AIC). Two-sample t-tests were run to investigate whether percent blubber content differed between spring and autumn within maturity/sex groups. When data did not fulfil the assumption of normality, a Mann–Whitney U-test was used instead. To investigate whether there were significant differences in blubber content between maturity/sex groups, and between years for adult seals in the autumn sample, one-way ANOVAs were used followed by Tukey’s tests with adjusted p-values to determine which groups differed significantly from each other. All statistical analyses were performed in R (version 3.5.2) and the level of significance (α) was set at 0.05.
Results
The seals in this study ranged in age from 0 (young of the year, n = 6) to 33 years. The sex ratio was 52 (52.5%) females and 47 (47.5%) males. Among the females, 46 (88.5%) were sexually mature whereas 29 (61.7%) males were mature (Table 1). Two of the 99 GITs (2%) were empty. All of the other GITs (97) contained prey remains: 70.7% of the stomachs; 81.8% of the small intestines and 86.9% of the large intestines had identifiable prey items. Otoliths were found in 91.9% of the GITs and 49.5% contained crustaceans (Table 2). The stomachs, small intestines and large intestines contained 31.8%, 45.2% and 23.02% of the otoliths, respectively. The relative proportions of various fish species found in the various gut segments can be found in Online Resource 2.
In total, 12 fish groups were recognized; seven of these were identified to the species level, while the other five were identified only to the level of the Family (Table 2). For crustaceans, 18 prey types were found; ten of these were identified to the species level, six to Genus and two to Order (Table 2). Only 0.15% of the otoliths and 0.02% of the invertebrate parts were unidentifiable. In addition to fish and crustaceans, algal fragments and small (< 2 mm), empty bivalve and gastropod shells were found in 10.1%, 58.6% and 14.1% of the seals’ GITs, respectively. Gastroliths were found in 61.6% of the seals. It is likely that bivalves and gastropods were secondary prey, as they are regular in the diet of pricklebacks (Pethon 2005), which were found in large numbers in the GITs of the seals. They were therefore not included in further analyses.
Most (55.6%) of the seals had ingested 2–4 different prey types (range 0–11; Fig. 2). There was no significant difference in the number of prey types consumed by different maturity/sex groups (Chi-square test, \({\chi }_{6}^{2}\) = 3.11, p = 0.7944).
Polar cod was the dominant prey type in terms of Bi and FOi (Tables 2, 3). Pricklebacks were the second most numerous fish prey type (Tables 2, 3) and the prey type with the second highest FOi (Table 2). In addition, several Atlantic fish species were found, the most important in terms of Bi (9.2%) and FOi (23.2%) was Atlantic cod. Other Atlantic species—Atlantic herring, blue whiting (Micromesistius poutassou) and capelin had FOi between 14.1 and 17.2%. Krill (Thysanoessa spp.) had the highest Ni (40.8%; Table 2).
Estimated lengths of 4 159 polar cod, from the stomachs and small intestines of 75 seals, ranged from 36.9 to 231.2 mm (Table 3; Fig. 3a). Kruskal Wallis rank sum test showed a significant difference between the maturity/sex groups (Kruskal Wallis test, H2 = 109.58, p < 0.0001) and a pairwise Wilcoxon rank sum test revealed that polar cod consumed by adult males were significantly larger than those consumed by adult females and juveniles (p ≤ 0.0001). Most of the polar cod belonged to YC 1 (58.7%) or YC 2 (28.4%), while approximately 4% belonged to YC 4 or higher (Fig. 3b). For samples collected in spring 2014 and 2015, polar cod was found only in small numbers. Krill made up more than half of the prey items numerically during the spring (Fig. 4a), but due to their small size, krill contributed little to the total biomass of the prey consumed by the ringed seals (Fig. 4b). Pricklebacks had the highest occurrence in terms of biomass for all years during spring (Fig. 4b). Because the sample size for spring was small, and some of the spring material was collected in Van Mijenfjorden (south of Isfjorden), more detailed exploration of diet composition was only conducted on the autumn samples (spring results are presented in Online Resource 3).
During autumn, polar cod dominated the diet in all years, except 2014, both in terms of numbers of items and biomass (Fig. 4c, d). High numbers of amphipods and krill were found in the autumn samples from 2014 and 2016, respectively (Fig. 4c), but again, due to their small size, these prey types contributed little in terms of biomass (Fig. 4d). Polar cod dominated the diet for all maturity/sex groups (Fig. 5a, b). Juveniles consumed more krill than adults, while adults of both sexes consumed more amphipods and pricklebacks compared to juveniles (Fig. 5a).
Relative frequencies of different prey types in the ringed seal diet on the west coast of Spitsbergen, Svalbard, during autumn (2014–2017), divided into maturity/sex groups (adult females, adult males and juveniles, by a numerical occurrence; b contribution by biomass; c numerical occurrence for prey types divided into Arctic and Atlantic prey classes and d contribution by biomass for prey types divided into Arctic and Atlantic
Arctic prey types dominated the diet of all seal maturity/sex groups, both by numbers and biomass (Fig. 5c, d). In terms of numbers, juvenile ringed seals consumed more Atlantic species (mainly krill) than adult seals (Fig. 5c). In terms of biomass, females consumed a slightly greater proportion of Atlantic species than males and juveniles (Fig. 5d).
Among the explanatory variables in the RDA (year, blubber content and maturity/sex group) year was the only significant explanatory variable retained after the forward selection procedure (RDA, F3 = 4.96, AIC = − 76.49, p ≤ 0.0050). This variable explained 19.1% of the total variation in diet composition (Fig. 6). The first two axes of the bi-plot (Fig. 6) explained 18.9% of the constrained variation in the model. Seals sampled in 2014 and 2015 consumed a higher biomass of sculpins and pricklebacks compared with the seals in 2016 and 2017. The highest biomasses of Atlantic cod and polar cod were found in GITs from 2016 and 2017, respectively (Fig. 6).
Redundancy analysis (RDA) bi-plot for biomass of selected prey species (blue) as response variables (for individual seals (grey circles)) by year (red). The independent predictor explained 19.1% of the variance in biomass (Hellinger-transformed) seen in the dependent variables. Prickle pricklebacks, Sculp sculpins, Atl.C Atlantic cod, Pol.C polar cod
Blubber content (%) was calculated for 95 seals (Fig. 7); four samples lacked data on either body- mass or length. Blubber content was significantly lower in the spring compared to the autumn for all maturity/sex groups (adult males, two-sample t-test, t27 = -6.63, p < 0.0001; juveniles, t21 = -4.03, p = 0.0006 and females, Mann–Whitney U-test, U = 4, p < 0.0001). There was a significant difference in blubber content between the maturity/sex groups during autumn (ANOVA, F2,65 = 6.91, p = 0.0019). The Tukey’s test revealed that juveniles had significantly lower blubber content than females (p = 0.0019). The sample size was too small to test for potential annual differences (during autumn) in blubber content of maturity/sex groups. However, a significant difference between years was found when pooling adult seals (ANOVA, F3,49 = 5.19, p = 0.0034); 2015 was significantly lower than 2016 (p = 0.0070) and 2017 (p = 0.0082).
Blubber content (%) of 95 ringed seals from the west coast of Spitsbergen, Svalbard (2014–2017), divided into sex and age grouping and compared between a seasons and b year (only autumn samples). Boxes contain values between the upper and the lower quartile and are divided by a line, representing the median value. Vertical lines through the boxes extend to the maximum and the minimum values (excluding outliers). Outliers are represented by dots, and are defined as values more than 1.5 times higher or lower than the upper and lower quartile, respectively. Numbers above boxes show sample sizes
Discussion
Ringed seals diets are known to vary seasonally, interannually and regionally (e.g. McLaren 1958; Thiemann et al. 2007). This suggests that the species is a generalist feeder that exhibits some capacity for dietary plasticity. The very marked environmental changes in the marine environment in Svalbard in recent decades have resulted in an Atlantification of both the fish and invertebrate communities. An isotopic study of diet in the region by Lowther et al. (2017) detected changes in the ringed seal whisker composition, and suggested that a change had taken place in either what ringed seals or their prey were consuming. The results of the current study suggest that the spring diet of ringed seals in this region might have become somewhat more varied following the environmental changes during the last decades. Autumn diets showed less change, though some few new Atlantic species were detected in this season as well. However, the ringed seals’ diet in Svalbard continues to be dominated by Arctic prey types, with polar cod being particularly important.
All methods of dietary analyses (DNA, fatty acids, stable isotopes, identification of hard-parts) have biases (Trites and Spitz 2018). The analyses of hard-parts from GITs used in this study represents only recent meals and otoliths and other materials are susceptible to partial or complete erosion when moving through the digestive system of a seal (Bowen and Harrison 1994). Gastric acid within the stomach is particularly corrosive (Christiansen et al. 2005). Dissolution rates are affected by the size of the otoliths and the robustness of hard-materials differs between species. Gadoid otoliths (e.g. polar cod and Atlantic cod) are generally quite robust while herring and capelin otoliths have higher dissolution rates (Christiansen et al. 2005; Grellier and Hammond 2006). This could have led to an overrepresentation in numbers and size of the former compared to the latter in this study. However, the proportions of both herring and capelin were higher in the samples from the large intestines than in the stomachs and small intestines, indicating that complete dissolution was probably not an issue. Meal size and feeding mode also have effects on the degree of erosion of prey hard-parts (Marcus et al. 1998; Grellier and Hammond 2005). For example, otoliths inside intact skull cases are more protected from erosion than otoliths that have come loose, e.g. by rough handling of fish by seals when feeding. It can also be assumed that skulls of fish species with strong bones, such as Atlantic cod, take longer to dissolve compared to those with more fragile skulls, e.g. pricklebacks, affording the otoliths differential protection. This can, to some degree, be accounted for by using species-specific recovery rates and digestion coefficients (Grellier and Hammond 2006). Such corrections were not used in this study because the number of otoliths found in each seal varied greatly, indicating a lot of variation in meal size and because such coefficients have not been calculated for ringed seals or their primary prey species. In the case of sculpins, blue whiting and eelpouts, relevant regressions for calculating fish length and mass from otolith length were lacking for the size of fishes consumed by the ringed seals. This adds an unknown degree of error to their relative contribution to the diet. However, due to their size and relatively small numbers, this was not thought to influence the overall results of this study markedly. Another issue when using otoliths to identify consumed fish species is that the head of the prey is not always consumed by the seals, especially for larger prey (Pierce and Boyle 1991). This results in a potential underestimation of the contribution of large fish prey such as Atlantic cod and saithe (Pollachius virens). The chitinous shells of crustaceans are relatively resistant to digestion within pinniped digestive systems (Sheffield et al. 2001; Staniland 2002), but in this study, samples from the small- and large intestine were broken in many small pieces. Thus, it was challenging to get a good estimate of numbers of telsons, heads or eye pairs. Whichever type of item was the most numerous was assumed to best represent a given type of prey consumed. Results on prey abundance and size of fish prey herein should be assessed with these biases in mind. A total of 30 different prey types were identified in the GITs of the ringed seals in the present study. However, only five prey types constituted more than 1% in terms of numbers and biomasses and most of the seals in this study had consumed between one and four different prey types, similar to the findings of Labansen et al. (2007) from Svalbard. This suggests that, in this area, ringed seal diets do include a variety of prey types, but that they show strong preferences for just a handful of species, especially polar cod.
This study clearly shows that polar cod remains the most important food source for ringed seal on the west coast of Spitsbergen during autumn, followed by other Arctic fish species in the prickleback and sculpin families (Renauld et al.2012; Fossheim et al. 2015). These findings are similar to previous studies of ringed seal diet in Svalbard and elsewhere across the Arctic (Lowry et al. 1980; Gjertz and Lydersen 1986; Lydersen et al. 1989; Węsławski et al. 1994; Siegstad et al. 1998; Wathne et al. 2000; Holst et al. 2001; Labansen et al. 2007, 2011). Polar cod in and around Svalbard are dispersed in the water column according to age class; smaller, younger fish (YC 1 and 2) are found in shallow water, often associated with sea ice, whereas older fish are more pelagic and reside at greater depths (Falk-Petersen et al. 1986; Lønne and Gulliksen 1989; Renaud et al. 2012). A similar size- and age-related distribution pattern in the water column has been documented for pricklebacks (Eriksen et al. 2012). Most of the polar cod consumed by seals in this study belonged to YC 1 and 2, similar to previous studies of ringed seal diet in Svalbard (Gjertz and Lydersen 1986; Węsławski et al. 1994; Labansen et al. 2007). This is consistent with observations of Svalbard ringed seals feeding mostly in the upper part of the water column where these young polar cod live (Gjertz et al. 2000; Wathne et al. 2000; Hamilton et al. 2015, 2016). The fact that adult males consumed larger polar cod than adult females and juveniles in this study suggests that they might be foraging deeper in the water column. However, comparing proportional biomasses of polar cod in the diet in this study (Bfish = 62%) with Labansen et al. (2007; Bfish = 77%) suggests that ringed seals might be eating less polar cod now compared to a decade ago. Additionally, the sizes of polar cod consumed in the two study periods further suggests that ringed seals are consuming polar cod of lower average weight. The energy content of polar cod increases with body size (Lawson et al. 1998; Harter et al. 2013), which means that seals in the current study probably consume polar cod with somewhat lower average caloric value than a decade ago. Similar to other Arctic species that have life cycles that include a sympagic phase, polar cod are at risk in a warming Arctic (Fossheim et al. 2015). In the last two decades polar cod have declined markedly in the Barents Sea region (Skaret et al. 2018, also see MOSJ https://www.mosj.no/en/fauna/marine/polar-cod.html).
Pricklebacks were the dominant prey type during spring in terms of biomass. Overall, it was the second most important prey type in terms of biomass and FOi and the second most numerous fish prey type. The otoliths of these fishes are small and hard to distinguish between species. Species of pricklebacks known to reside in Svalbard include: Lumpenus lampraeteformis, L. fabricii, Leptoclinus maculatus and Anisarhus medius (Pethon 2005; Eriksen et al. 2012). Labansen et al. (2007) suggested that pricklebacks in the diet of ringed seals in Svalbard waters might be a local phenomenon in Forlandsundet and St Jonsfjorden, because these fishes did not contribute substantially to the ringed seal diet in previous studies or in other fjord systems in their study. The current study shows that these fish are also important for ringed seals in Isfjorden and Van Mijenfjorden, which are to the south of Forlandsundet and St Jonsfjorden.
This is the first time that blue whiting has been recorded as prey for ringed seals. This fish is an Atlantic species with a distribution that stretches across much of the Barents Sea (Pethon 2005; Dolgov et al. 2010). An increase in the presence of this species around Svalbard is thought to be connected with increased inflows of AW in this area (Bergstad et al. 2018).
Krill was the dominant crustacean found in the GITs of the ringed seals from Svalbard. In the Barents Sea, krill are associated with AW and their abundance around Svalbard is highly variable from year to year, largely correlated with the variations in the inflow of AW (Dalpadado and Skjoldal 1996; Ellingsen et al. 2008). Crawford et al. (2015) found that FOi of crustaceans had decreased over time in ringed seal diet in the Bering and Chukchi seas off Alaska. In contrast, the FOi (49.5%) and Ni (47.7%) of crustaceans in the present study were higher than what Labansen et al. (2007) found in Svalbard 15 years ago (FOi = 38.2%; Ni = 1.88%). Furthermore, most of the krill in the study were consumed by seals during spring. Labansen et al.’s (2007) study was conducted during spring 2002–2004, but these authors found only eight individual krill in their large ringed seals collection. Tracking results suggest that younger seals spend more time further away from glacier fronts than adult seals, probably due to competitive exclusion (Hamilton et al. 2016). If juveniles feed further out in the fjords, where the influence of AW is greater than at the glacier fronts, this could explain why juveniles had higher relative numbers of krill in their diet during autumn than adult seals. Ringed seal feeding studies conducted at the ice edge in the Northeast Barents Sea, suggest that ringed seals display a strong preference for polar cod, regardless of its relative availability (Wathne et al. 2000), so the dominance of krill in terms of numbers in the spring diet during 2014 and 2015 in this study is noteworthy. However, given the lack of knowledge regarding actual availability of the various potential prey types, it is not possible to determine the degree of selectivity that the ringed seals might be displaying. The results herein for spring are likely linked to inter-annual variation in Atlantic water influxes in the spring season, and hence krill vs polar cod densities, in the various years of this study.
In contrast to what was observed during spring by Labansen et al. (2007), no significant maturity and sex related differences were detected by the multivariate analysis of diet composition during autumn in the current study. The choice to explore diet composition in terms of biomass, as opposed to prey counts (Labansen et al. 2007), was made in the current study because biomass was considered to better represent the relative importance of each prey type. This was especially relevant considering the low counts, but high biomass contribution, of Atlantic cod and the high counts but low biomass contribution of krill to the diet composition of the ringed seal in this study.
The RDA showed that neither blubber content, nor maturity/sex contributed significantly to explaining variation in diet composition. This is in contrast with what has been observed in another Arctic seal present in the Barents Sea, the harp seal (Pagophilus groenlandicus; Lindstrøm et al. 2013). However, it should be pointed out that the RDA results were sensitive to the choice of data transformation (log, Hellinger, square root and Chi-square distance). Regardless of which transformation was used, year was significant, and maturity/sex group was not. Blubber content was on the border of being significant when applying log and square root-transformed data (RDA, F1 = 2.46, AIC = 404.09, p = 0.0800). Prey availability may be considered a latent variable inferred by the predictor variable “year”, i.e. the inter-annual variation in diet composition (during autumn) is most likely a result of changes in prey availability rather than changes in prey preference. It appears that ringed seals prefer polar cod but will feed opportunistically on other types of prey when necessary (also see Wathne et al 2000).
Blubber content of the seals in this study was lower during spring compared to autumn, especially for sexually mature seals. This is a normal seasonal pattern for all Arctic phocid seals and many other Arctic animals (Ryg et al. 1990a). The size of the spring sample in this study is low and unevenly distributed over the years, and more importantly over the various months grouped into the “spring” sample. Due to small sample sizes in the only study that has reported blubber contents from Svalbard in autumn (Ryg et al. 1990a), it is impossible to draw firm conclusions regarding longer-term temporal trends, but seals from all groups in the autumn sample in the current study had higher average blubber contents than the ringed seal study by Ryg et al. (1990a). This is consistent with Crawford et al. (2015), who reported an increase in ringed seal blubber thickness in recent years (2003–2012) compared to earlier (1975–1984), in areas around the Bering Strait. These findings are in contrast to the temporal patterns found by Ferguson et al. (2017) in Hudson Bay from 2004 to 2013, where condition has declined. This highlights the importance of studying various ringed seal populations across the Arctic. On first reflection body condition of ringed seals in Svalbard does not seem to be a cause of concern for the local population. However, paradoxically, females with higher blubber content than normal during autumn might in fact be a warning sign. The largest energy output in an adult ringed seal female’s annual cycle is lactation (Lydersen and Kovacs 1999). In recent years, the snow and ice condition in Svalbard have been unfavourable for ringed seal’s completing the lactation period in many years, largely due to high levels of surface predation on pups. Consistently, 2015 stands out as a year when adult seals were in a poorer condition during autumn than the others years. In this year the ice-cover in Isfjorden and the neighbouring fjords, Van Mijenfjorden and St Jonsfjorden in March to May was greater during 2015 than the other years (Skoglund pers. comm.). These favourable ice-conditions likely resulted in more of the seals going through a normal breeding season with lactation and territorial defence periods, resulting in a lower, more normal, body condition the coming autumn. Alternatively, the spring diet for this particular year, with its’ large numbers of krill might have resulted in somewhat thinner animals. Krill (2.78–5.04 kJ g−1; Lowry et al. 1980) and other Atlantic species, such as Atlantic cod (4.2 kJ g−1; Lawson et al. 1998) have lower lipid contents compared to the key Arctic species in the ringed seals’ diet such as polar cod (5.90 kJ g−1; Lowry et al. 1980; 4.70 kJ g−1; Elliot and Gaston 2008) and pricklebacks (4.97 kJ g−1; Elliot and Gaston 2008).
Lowther et al. (2017) suggested that a dietary shift had likely taken place for ringed seals over recent decades based on isotopic analyses of the whiskers. The current study found that the contribution of polar cod to the diet in terms of biomass has declined somewhat (Bfish = 61.9%), compared to what was found in this area in 2002–2004 (Bfish = 77.2%; Labansen et al. 2007), while the importance of pricklebacks has increased (Bfish = 24.1% vs. Bfish = 13.8%). Additionally, a new Atlantic species has been found in the diet (blue whiting) and other Atlantic species have relatively higher importance, e.g. krill and capelin. However, it is also likely that the increase in AW around Svalbard has affected the diet of the main prey of ringed seals, i.e. polar cod. This fish species has been described as an opportunistic feeder (Ajiad and Gjøsæter 1990) and it might be eating more Atlantic crustacean species, which are increasing in abundance in Svalbard (Dalpadado et al. 2016).
Recent tracking studies of ringed seals in Svalbard have shown that this species has altered its space use patterns following the marked sea ice declines that have occurred in the region, likely as a response to the increased influx of AW in this area (Hamilton et al. 2016, 2019a). Especially adult animals now spent virtually all of their time at tidewater glacier fronts, where polar cod concentrate (Lydersen et al. 2014; Fey and Węsławski 2017). Both ringed seals and polar cod appear to be retracting into Arctic glacial refugia where cold water conditions persist.
The sample size in this study is small compared to many studies of pinniped diets. This was due to the fact that a targeted hunt on ringed seals for research purposes was not deemed ethically acceptable at this time because the ringed seal population in Svalbard is thought to be declining due to reduction in their breeding habitat. Thus, samples were only available from a low-level sport hunt conducted by Svalbard residents. This meant that analyses of spring diets were limited to descriptive assessments, and that samples for maturity/sex groups, even in the larger autumn sample had to be pooled for some analyses. Despite these short-comings, several important results emerge. Firstly, Atlantic species, especially gadoids like Atlantic cod, Atlantic herring and krill have increased in frequency and biomass in the diet of ringed seals in Svalbard (see Węsławski et al. 1994 and Labasen et al. 2007 for reference). This suggests some degree of plasticity in responding to changing availability of these prey types. Secondly, ringed seals still display a strong preference for Arctic species, especially polar cod. This is a concern for the future of the ringed seal population in areas where polar cod is in decline, such as the Barents Sea. The ringed seal’s ability to adapt to further Atlantification of Svalbard is unknown, but the major changes to both their breeding habitat and their preferred prey base are cause for concern.
References
ACIA (2005) Arctic climate impacts assessment. Cambridge University Press, Cambridge
Ajiad AM, Gjøsæter H (1990) Diet of polar cod, Boreogadus saida, in the Barents Sea related to fish size and geographical distribution. ICES CM 1990 G:48.1–9
Berge J, Heggland K, Lønne OJ, Cottier F, Hop H, Gabrielsen GW, Nøttestad L, Misund OA (2015) First records of Atlantic mackerel (Scomber scombrus) from the Svalbard Archipelago, Norway, with possible explanations for the extension of its distribution. Arctic 68:54–61
Bergstad OA, Johannesen E, Høines Å, Ellingsen KE, Lien VS, Byrkjedal I, Yoccoz NG, Tveraa T, Wienerroither R, Langhelle G, de Lange WT (2018) Demersal fish assemblages in the boreo-Arctic shelf waters around Svalbard during the warm period 2007–2014. Polar Biol 41:125–142
Beszczynska-Möller A, Fahrbach E, Schauer U, Hansen E (2012) Variability in Atlantic water temperature and transport at the entrance to the Arctic Ocean, 1997–2010. ICES J Mar Sci 69:852–863
Bilt W, Bakke JB, Smedsrud LH, Sund M, Schuler TV, Westermann S, Wong WK, Sandven S, Simpson MJR, Skogen MD, Pavlova O, Ravndal O, Risebrobakken B, Saloranta Tm, Mezghani A, Nilsen F, Nilsen JEO, Nilsen IB, Kierulf H, Kohler J, Li H, Lutz J, Melvold K, Gjelten HM, Gundersen J, Isaksen K, Jaedicke C, Dobler A, Engeset R, Frauenfelder KR, Gerland S, Christiansen HH, Børsheim KY, Breivik Ø, Breili K, Borstad CP, Bogen J, Benestad R, Beldring S, Andresen J, Adakudlu M, Førland E, Hisdal H, Mayer S, Hanssen-Bauer I, Sandø AB, Sorteberg A (2019) Climate in Svalbard 2100. Norwegian Centre for Climate Services Reports. https://hdl.handle.net/1956/19136 Accessed 23 Apr 2019
Błaszczyk M, Jania JA, Hagen JO (2009) Tidewater glaciers of Svalbard: recent changes and estimates of calving fluxes. Pol Polar Res 30:85–142
Bowen WD, Harrison GD (1994) Offshore diet of grey seals Halichoerus grypus near Sable Island, Canada. Mar Ecol Prog Ser 112:1–11
Brand M, Fischer P (2016) Species composition and abundance of the shallow water fish community of Kongsfjorden, Svalbard. Polar Biol 39:2155–2167
Christiansen JS, Gamst Moen A-G, Hansen T, Nilssen KT (2005) Digestion of capelin, Mallotus villous (Müller), herring, Clupea harengus L., and polar cod, Boreogadus saida (Lepechin), otoliths in a simulated seal stomach. ICES J Mar Sci 62:86–92
Corfield J (2000) The effects of acid sulphate run-off on a subtidal estuarine macrobenthic community in the Richmond River, NSW, Australia. ICES J Mar Sci 57:1517–1523
Cottier FR, Tverberg V, Inall M, Svendsen H, Nilsen F, Griffiths C (2005) Water mass modification in an Arctic fjord through cross-shelf exchange: the seasonal hydrography of Kongsfjorden. Svalbard J Geophys Res 110:C12005. https://doi.org/10.1029/2004JC002757
Crawford JA, Quakenbush LT, Citta JJ (2015) A comparison of ringed and bearded seal diet, condition and productivity between historical (1975–1984) and recent (2003–2012) periods in the Alaskan Bering and Chukchi seas. Prog Oceanogr 136:133–150
Dalpadado P, Skjoldal HR (1996) Abundance, maturity and growth of the krill species, Thysanoessa inermis and T. longicaudata in the Barents Sea. Mar Ecol Prog Ser 144:175–183
Dalpadado P, Hop H, Ronning J, Pavlov V, Sperfeld E, Buchholz F, Rey A, Wold A (2016) Distribution and abundance of euphausiids and pelagic amphipods in Kongsfjorden, Isfjorden and Rijpfjorden (Svalbard) and changes in their relative importance as key prey in a warming marine ecosystem. Polar Biol 39:1765–1784
Dolgov AV, Johannesen E, Heino M, Olsen E (2010) Trophic ecology of blue whiting in the Barents Sea. ICES J Mar Sci 67:483–493
Drinkwater KF (2005) The response of Atlantic cod (Gadus morhua) to future climate change. ICES J Mar Sci 62:1327–1337
Ellingsen IH, Dalpadado P, Slagstad D, Loeng H (2008) Impact of climatic change on the biological production in the Barents Sea. Clim Change 87:155–175
Elliott KH, Gaston AJ (2008) Mass–length relationships and energy content of fishes and invertebrates delivered to nestling thick-billed murres Uria lomvia in the Canadian Arctic, 1981–2007. Mar Ornithol 36:25–34
Enckell PH (1980) Kräftdjur. [Crustaceans] Bokförlaget Signum, Lund, Sweden
Eriksen E, Prokhorova T, Johannesen E (2012) Long term changes in abundance and spatial distribution of pelagic Agonidae, Ammodytidae, Liparidae, Cottidae, Myctophidae and Stichaeidae in the Barents Sea. In: Ali M (ed) Diversity of ecosystems. In Tech, Rijeka, pp 109–126
Falk-Petersen I-B, Frivoll V, Gulliksen B, Haug T (1986) Occurrence and size/age relations of polar cod, Boreogadus saida (Lepechin), in Spitsbergen coastal waters. Sarsia 71:235–245
Fey DP, Węsławski JM (2017) Age, growth rate, and otolith growth of polar cod (Boreogadus saida) in two fjords of Svalbard, Kongsfjorden and Rijpfjorden. Oceanologia 59:576–584
Ferguson SH, Young BG, Yurkowski DJ, Anderson R, Willing C, Nielsen O (2017) Demographic, ecological, and physiological responses of ringed seals to an abrupt decline in sea ice availability. PeerJ 5:e2957. https://doi.org/10.7717/peerj.2957
Finley KJ, Miller GW, Davis RA, Koski WR (1983) A distinctive large breeding population of ringed seals (Phoca hispida) inhabiting the Baffin Bay pack ice. Arctic 36:162–173
Fossheim M, Primicerio R, Johannesen E, Ingvaldsen RB, Aschan MM, Dolgov AV (2015) Recent warming leads to a rapid borealization of fish communities in the Arctic. Nat Clim Change 5:673–677
Freitas C, Kovacs KM, Ims RA, Fedak MA, Lydersen C (2008) Ringed seal post-moulting movement tactics and habitat selection. Oecologia 155:193–204
Gjertz I, Lydersen C (1986) The ringed seal (Phoca hispida) spring diet in northwestern Spitsbergen, Svalbard. Polar Res 4:53–56
Gjertz I, Kovacs KM, Lydersen C, Wiig Ø (2000) Movements and diving of adult ringed seals (Phoca hispida) in Svalbard. Polar Biol 23:651–656
Grellier K, Hammond PS (2005) Feeding method affects otolith digestion in captive gray seals: implications for diet composition estimation. Mar Mamm Sci 21:296–306
Grellier K, Hammond PS (2006) Robust digestion and passage rate estimates for hard parts of grey seal (Halichoerus grypus) prey. Can J Fish Aquat Sci 63:1982–1998
Hamilton CD, Lydersen C, Ims RA, Kovacs KM (2015) Predictions replaced by facts: a keystone species’ behavioural responses to declining Arctic sea ice. Biol Lett 11:20150803. https://doi.org/10.1098/rsbl.2015.0803
Hamilton CD, Lydersen C, Ims RA, Kovacs KM (2016) Costal habitat use by ringed seals Pusa hispida following a regional sea-ice collapse: importance of glacial refugia in a changing Arctic. Mar Ecol Prog Ser 545:261–277
Hamilton CD, Kovacs KM, Lydersen C (2019) Sympatric use of a glacial fjord by two Arctic endemic seals. Mar Ecol Prog Ser 615:205–220
Hamilton CD, Vacquie-Garcia J, Kovacs KM, Ims RA, Kohler J, Lydersen C (2019) Contrasting changes in space use induced by climate change in two Arctic marine mammal species. Biol Lett 15:20180834. https://doi.org/10.1098/rsbl.2018.0834
Hammill MO, Lydersen C, Ryg M, Smith TG (1991) Lactation in the ringed seal (Phoca hispida). Can J Fish Aquat Sci 48:2471–2476
Härkönen T (1986) Guide to the Otoliths of the Bony Fishes of the Northeast Atlantic. Danbiu Aps, Hellerup
Hartley CH, Fisher J (1936) The marine foods of birds in an inland fjord region in west Spitsbergen. J Anim Ecol 5:370–389
Harter BB, Elliott KH, Divoky GJ, Davoren GK (2013) Arctic cod (Boreogadus saida) as prey: fish length-energetics relationships in the Beaufort Sea and Hudson Bay. Arctic 66:191–196
Holst M, Stirling I, Hobson KA (2001) Diet of ringed seals (Phoca hispida) on the east and west sides of the North Water Polynya, northern Baffin Bay. Mar Mamm Sci 17:888–908
Hop H, Gjøsæter H (2013) Polar cod (Boreogadus saida) and capelin (Mallotus villosus) as key species in marine food webs of the Arctic and the Barents Sea. Mar Biol Res 9:878–894
Hyslop EJ (1980) Stomach contents analysis: a review of methods and their application. J Fish Biol 17:411–429
IPCC (2014) Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assesssment Report of the Intergovernmental Panel on Climate Change. IPCC, Geneva, Switzerland. https://archive.ipcc.ch/pdf/assessment-report/ar5/syr/SYR_AR5_FINAL_full_wcover.pdf. Accessed 9 May 2019
Iversen M, Aars J, Haug T, Alsos IG, Lydersen C, Bachmann L, Kovacs KM (2013) The diet of polar bears (Ursus maritimus) from Svalbard, Norway, inferred from scat analysis. Polar Biol 36:561–571
Kortsch S, Primicerio R, Fossheim M, Dolgov AV, Aschan M (2015) Climate change alters the structure of arctic marine food webs due to poleward shifts of boreal generalists. Proc R Soc B 282:20151546–20151549. https://doi.org/10.1098/rspb.2015.1546
Kovacs K, Lydersen C, Overland J, Moore SE (2011) Impacts of changing sea-ice conditions on Arctic marine mammals. Mar Biodiv 41:181–194
Labansen AL, Lydersen C, Haug T, Kovacs KM (2007) Spring diet of ringed seals (Pusa hispida) from north-western Spitsbergen, Norway. ICES J Mar Sci 64:1246–1256
Labansen AL, Lydersen C, Levermann N, Haug T, Kovacs KM (2011) Diet of ringed seals (Pusa hispida) from Northeast Greenland. Polar Biol 34:227–234
Laidre KL, Stirling I, Lowry LF, Wiig Ø, Heide-Jørgensen MP, Ferguson SH (2008) Quantifying the sensitivity of Arctic marine mammals to climate-induced habitat change. Ecol Appl 18:97–125
Laidre KL, Stern H, Kovacs KM, Lowry L, Moore SE, Regehr EV, Ferguson SH, Wiig Ø, Boveng P, Angliss RP, Born EW, Litovka D, Quakenbush L, Lydersen C, Vongraven D, Ugarte F (2015) Arctic marine mammal population status, sea ice habitat loss, and conservation recommendations for the twenty-first century. Conserv Biol 29:724–737
Lawson JW, Magalhães AM, Miller EH (1998) Important prey species of marine vertebrate predators in the northwest Atlantic: proximate composition and energy density. Mar Ecol Prog Ser 164:13–20
Leclerc L-M, Lydersen C, Haug T, Bachmann L, Fisk A, Kovacs K (2012) A missing piece in the Arctic food web puzzle? Stomach contents of Greenland sharks sampled in Svalbard, Norway. Polar Biol 45:1197–1208
Legendre P, Legendre L (1998) Numerical ecology, 2nd, English edn. Elsevier, Amsterdam
Legendre P, Anderson MJ (1999) Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. Ecol Monogr 69:1–24
Legendre P, Gallagher ED (2001) Ecologically meaningful transformations for ordination of species data. Oecologia 129:271–280
Lepš J, Šmilauer P (2003) Multivariate analysis of ecological data using Canoco. Cambridge University Press, Cambridge
Lind S, Ingvaldsen RB (2012) Variability and impacts of Atlantic Water entering the Barents Sea from the north. Deep Sea Res Part I 62:70–88
Lind S, Ingvaldsen RB, Furevik T (2018) Arctic warming hotspot in the northern Barents Sea linked to declining sea-ice import. Nat Clim Change 8:634–639
Lindbäck K, Kohler J, Pettersson R, Nuth C, Langley K, Messerli A, Vallot D, Matsuoka K, Brandt O (2018) Subglacial topography, ice thickness, and bathymetry of Kongsfjorden, northwestern Svalbard. Earth Syst Sci Data 10:1769–1781
Lindstrøm U, Nilssen KT, Pettersen LMS, Haug T (2013) Harp seal foraging behaviour during summer around Svalbard in the northern Barents Sea: diet composition and the selection of prey. Polar Biol 36:305–320
Lone K, Hamilton C, Aars J, Lydersen C, Kovacs K (2019) Summer habitat selection by ringed seals (Pusa hispida) in the drifting sea ice of the northern Barents Sea. Polar Res 38:3483. https://doi.org/10.33265/polar.v38.3483
Lønne OJ, Gulliksen B (1989) Size, age, and diet of polar cod, Boreogadus saida (Lepechin 1773) in ice covered waters. Polar Biol 9:187–191
Lowry LF, Fay FH (1984) Seal eating by walruses in the Bering and Chukchi Seas. Polar Biol 3:11–18
Lowry LF, Frost KJ, Burns JJ (1980) Variability in the diet of ringed seals, Phoca hispida, in Alaska. Can J Fish Aquat Sci 37:2254–2261
Lowther AD, Fisk A, Kovacs KM, Lydersen C (2017) Interdecadal changes in the marine food web along the west Spitsbergen coast detected in the stable isotope composition of ringed seal (Pusa hispida) whiskers. Polar Biol 40:2027–2033
Lydersen C (1998) Status and biology of ringed seals (Phoca hispida) in Svalbard. NAMMCO Sci Publ 1:46–62
Lydersen C, Gjertz I (1987) Population parameters of ringed seals (Phoca hispida) Schreber, 1775) in the Svalbard area. Can J Zool 65:1021–1027
Lydersen C, Smith TG (1989) Avian predation on ringed seal Phoca hispida pups. Polar Biol 9:489–490
Lydersen C, Kovacs KM (1999) Behaviour and energetics of ice-breeding, North Atlantic phocid seals during the lactation period. Mar Ecol Prog Ser 187:265–281
Lydersen C, Gjertz I, Węsławski JM (1989) Stomach contents of autumn-feeding marine vertebrates from Hornsund, Svalbard. Polar Rec 25:107–114
Lydersen C, Assmy P, Falk-Petersen S, Kohler J, Kovacs KM, Reigstad M, Steen H, Strøm H, Sundfjord A, Varpe Ø, Walczowski W, Węsławski JM, Zajaczkowski M (2014) The importance of tidewater glaciers for marine mammals and seabirds in Svalbard, Norway. J Mar Syst 129:452–471
Marcus J, Bowen WD, Eddington JD (1998) Effects of meal size on otolith recovery from fecal samples of gray and harbor seal pups. Mar Mamm Sci 14:789–802
McLaren IA (1958) The biology of the ringed seal, Phoca hispida, in the eastern Canadian Arctic. Bull Fish Res Board Can 118:1–97
McLaren IA, Smith TG (1985) Population ecology of seals: retrospective and prospective views. Mar Mammal Sci 1:54–83
Melnikov VV, Zagrebin IA (2005) Killer whale predation in coastal waters of the Chukotka Peninsula. Mar Mamm Sci 21:550–556
Misund OA, Heggland K, Skogseth R, Falck E, Gjøsæter H, Sundet J, Watne J, Lønne OJ (2016) Norwegian fisheries in the Svalbard zone since 1980. Regulations, profitability and warming waters affect landings. Polar Sci 10:312–322
Nuth C, Kohler J, König M, von Deschwanden A, Hagen JO, Kääb A, Moholdt G, Petterson R (2013) Decadal changes from a multi-temporal glacier inventory of Svalbard. Cryosphere 7:1603–1621
Nuth C, Moholdt G, Kohler J, Hagen JO, Kääb A (2010) Svalbard glacier elevation changes and contribution to sea level rise. J Geophys Res 115:F01008. https://doi.org/10.1029/2008JF001223
Overland JE, Wang M (2010) Large-scale atmospheric circulation changes associated with the recent loss of Arctic sea ice. Tellus 62A:1–9
Pavlova O, Gerland S, Hop H (2019) Changes in sea-ice extent and thickness in Kongsfjorden, Svalbard (2003–2016). In: Hop H, Wiencke C (eds) The ecosystem of Kongsfjorden, Svalbard. Springer, Cham, pp 49–104
Pethon P (2005) Aschehougs store fiskebok. Aschehoug Forlag, Oslo
Pierce GJ, Boyle PR (1991) A review of methods for diet analysis of piscivorous marine mammals. Oceanogr Mar Biol 29:409–486
Reeves RR (1998) Distribution, abundance and biology of ringed seals (Phoca hispida): an overview. NAMMCO Sci Publ 1:9–45
Renaud PE, Berge J, Varpe Ø, Lønne OJ, Nahrgang J, Ottesen C, Hallanger I (2012) Is the poleward expansion by Atlantic cod and haddock threatening native polar cod, Boreogadus saida? Polar Biol 35:401–412
Renaud PE, Daase M, Banas NS, Gabrielsen TM, Søreide JE, Varpe Ø, Cottier F, Falk-Petersen S, Halsband C, Vogedes D, Heggland K, Berge J (2018) Pelagic food-webs in a changing Arctic: a trait-based perspective suggests a mode of resilience. ICES J Mar Sci 75:1871–1881
Ryg M, Øritsland NA (1991) Estimates of energy expenditure and energy consumption of ringed seals (Phoca hispida) throughout the year. Polar Res 10:595–601
Ryg M, Smith TG, Øritsland NA (1988) Thermal significance of the topographical distribution of blubber in ringed seals (Phoca hispidia). Can J Fish Aquat Sci 45:985–992
Ryg M, Lydersen C, Markussen NH, Smith T, Øritsland NA (1990a) Estimating the blubber content of phocid seals. Can J Fish Aquat Sci 47:1223–1227
Ryg M, Smith TG, Øritsland NA (1990b) Seasonal changes in body mass and body composition of ringed seals (Phoca hispida) on Svalbard. Can J Zool 68:470–475
Ryg M, Smith TG, Øritsland NA (1991) Seasonal and developmental changes in reproductive organs of male ringed seals (Phoca hispida) in the Svalbard area. J Zool 224:93–100
Scheffer VB (1967) Standard measurements of seals. J Mamm 48:459–462
Sheffield G, Fay FH, Feder H, Kelly BP (2001) Laboratory digestion of prey and interpretation of walrus stomach contents. Mar Mamm Sci 17:310–330
Siegstad H, Neve PB, Heide-Jørgensen MP, Härkönen T (1998) Diet of the ringed seal (Phoca hispida) in Greenland. NAMMCO Sci Publ 1:229–241
Simmonds MP, Isaac SJ (2007) The impacts of climate change on marine mammals: early signs of significant problems. Oryx 41:19–26
Skaret G, Prozorkevich D, Gjøsæter H, Bogstad B (2018) Influence of ecosystem changes on harvestable resources at high latitudes. The Proceedings of the 18th Russian-Norwegian Symposium. IMR/PINRO Joint Report Series, Murmansk, Russia
Smith TG (1976) Predation of ringed seal pups (Phoca hispida) by the arctic fox (Alopex lagopus). Can J Zool 54:1610–1616
Smith TG, Hammill MO, Taugbol T (1991) A review of the developmental, behavioural and physiological adaptations of the ringed seal, Phoca hispida, to life in the arctic winter. Arctic 44:124–131
Søreide JE, Carroll ML, Hop H, Ambrose WG, Hegseth EN, Falk-Petersen S (2013) Sympagic-pelagic-benthic coupling in Arctic and Atlantic waters around Svalbard revealed by stable isotopic and fatty acid tracers. Mar Biol Res 9:831–850
Spielhagen RF, Werner K, Sørensen SA, Zamelszyk K, Kandiano E, Budeus G, Husum K, Marchitto TM, Hald M (2011) Enhanced modern heat transfer to the Arctic by warm Atlantic water. Science 331:450–453
Staniland IJ (2002) Investigating the biases in the use of hard prey remains to identify diet composition using Antarctic fur seals (Arctocephalus gazella) in captive feeding trials. Mar Mamm Sci 18:223–243
Stirling I, Øritsland NA (1995) Relationships between estimates of ringed seal and polar bear populations in the Canadian Arctic. Can J Fish Aquat Sci 52:2594–2612
Teilmann J, Kapel FO (1998) Exploitation of ringed seals (Phoca hispida) in Greenland. NAMMCO Sci Publ 1:130–151
Thiemann GW, Iverson SJ, Stirling I (2007) Variability in the blubber fatty acid composition of ringed seals (Phoca hispida) across the Canadian Arctic. Mar Mamm Sci 23:241–261
Trites AW, Spitz J (2018) Diet. In: Würsig B, Kovacs KM (eds) Encyclopedia of Marine Mammals, 3rd edn. Academy Press, Cambridge, pp 255–259
Tverberg V, Nøst OA, Lydersen C, Kovacs KM (2014) Winter sea ice melting in the Atlantic Water subduction area, Svalbard Norway. J Geophys Res C Oceans 119:5945–5967
Tynan CT, DeMaster DP (1997) Observations and predictions of arctic climate change: potential effects on marine mammals. Arctic 50:308–322
Vihtakari M, Welcker J, Moe B, Chaste O, Tartu S, Hop H, Bech C, Descamps S, Gabrielsen GW (2018) Black-legged kittiwakes as messengers of Atlantification in the Arctic. Sci Rep 8:1178. https://doi.org/10.1038/s41598-017-19118-8
Wang M, Overland JE (2009) A sea ice free summer Arctic within 30 years? Geophys Res Lett 36:L07502. https://doi.org/10.1029/2009GL037820
Wathne JA, Haug T, Lydersen C (2000) Prey preferences and niche overlap of ringed seals Phoca hispida and harp seals P. groenlandica in the Barents Sea. Mar Ecol Prog Ser 194:233–239
Węsławski JM, Ryg M, Smith TG, Oritsland NA (1994) Diet of ringed seals (Phoca hispida) in a fjord of West Svalbard. Arctic 47:109–114
Wiig Ø, Derocher AI, Belikov SE (1999) Ring seal (Phoca hispida) breeding in the drifting pack ice of the Barents Sea. Mar Mamm Sci 15:595–598
Windsland K, Lindstrøm U, Nilssen KT, Haug T (2007) Relative abundance and size composition of prey in the common minke whale diet in selected areas of the northeast Atlantic during 2000–04. J Cetacean Res Manag 9:167–178
Acknowledgements
Open Access funding was provided by Norwegian Polar Institute. This study was financed by the Svalbard Environmental Protection Fund and the Norwegian Polar Institute. We thank hunters that contributed to the collection programme (Martin Munck, Karina Bernlow, Tommy Jordbrudal, Eike Stubner and Tommy Sandal). We also thank: Lotta Lindblom for assisting with the lab work and helping with identification of prey species; Magnus Andersen for his contributions to the generation of the biological data on the seals and for handling/transport of samples; Anders Skoglund for providing the map of the sampling region; Charmain Hamilton for sharing her extensive R knowledge; Sebastian Prati for valuable input on statistics; and Heidi Gabrielsen for helping with identification of some of the crustacean species. Reviews by the Editor (Dr Dieter Piepenburg) and Dr Garry Stenson helped us improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bengtsson, O., Lydersen, C., Kovacs, K.M. et al. Ringed seal (Pusa hispida) diet on the west coast of Spitsbergen, Svalbard, Norway: during a time of ecosystem change. Polar Biol 43, 773–788 (2020). https://doi.org/10.1007/s00300-020-02684-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-020-02684-5