Abstract
The phenology and habitat selection of polar bear (Ursus maritimus) maternity dens may shift over time in response to changing environmental conditions. We compared maternity den phenology and habitat characteristics using satellite telemetry data from adult female polar bears from the Baffin Bay (BB) (n = 16 dens; 2009–2015) and Kane Basin (KB) subpopulations (n = 3 dens; 2012–2015) to previously published maternity den data from 1991 to 1997 (BB n = 8 dens; KB n = 3 dens). BB maternity denning duration decreased from a mean of 194.1 days (SD = 21.0 days, n = 8) in the 1990s, to a mean of 167.1 days (SD = 27.6 days, n = 16; p = 0.017) in the 2000s. Delayed den entry accounted for shorter denning durations (1990s entry date \(\bar{x}\) = 7 September; 2000s entry date \(\bar{x}\) = 5 October; p = 0.018). For dens habitat characteristics of which could be measured, BB maternity dens in the 2000s occurred at higher elevations (\(\bar{x}\) = 707.0 m, SD = 284.9 m, n = 15; p = 0.003) and greater slopes (\(\bar{x}\) = 23.1°, SD = 7.4°; p = 0.003) than the 1990s (elevation \(\bar{x}\) ± SD = 351.3 ± 194.5 m, n = 8; slope \(\bar{x}\) ± SD = 11.9 ± 6.4°). Aspect also significantly differed between the 1990s (\(\bar{x}\) = 51.3°) and 2000s BB maternity dens (\(\bar{x}\) = 199.7°; Watson’s U 2 p = 0.042). KB dens were not statistically compared due to low sample size (n = 3 dens in both periods). Shifts in sea ice phenology and snow availability may explain the observed changes.
Similar content being viewed by others
Introduction
Shifts in phenology and distribution are among the most readily observable biological responses to habitat modifications brought on by climate change (Sparks and Menzel 2002; Walther et al. 2002; Parmesan and Yohe 2003; Thackeray et al. 2010). Phenological traits characterize the timing of important life history events and are intimately associated with seasonal cues such as variation in temperature (Andrewartha and Birch 1954; Badeck et al. 2004). There is mounting evidence that warmer ambient mean temperatures are altering the seasonal phenology of many systems, such as earlier leaf budding and egg-laying in the spring (Parmesan and Yohe 2003; Root et al. 2003). Habitat-use and population distributions are similarly governed by abiotic factors such as temperature and precipitation, and some species are shifting their ranges polewards and to higher elevations in response to rising temperatures (Peters 1992; Parmesan and Yohe 2003; Root et al. 2003; Parmesan 2006; Parry et al. 2007).
The ecological impact of climate change is expected to be most pronounced at high latitudes (Root et al. 2003), including the Arctic where annual mean temperatures are rapidly increasing (IPCC 2014). Physiological constraints of Arctic species limit their ability to expand their range (Parmesan 2006; Williams et al. 2010), and ice-adapted organisms are facing extensive habitat loss (Laidre et al. 2008; Wassmann et al. 2011). Since the 1970s, the extent of the Arctic sea ice has declined by an average of 3.8% per decade and wintertime ice has thinned by 2 m (Vaughan et al. 2013). Furthermore, the total volume of sea ice has been dramatically reduced year-round (IPCC 2014), and break-up is occurring earlier in the year while freeze-up is occurring later in the autumn (Laidre et al. 2015a). For Arctic marine mammals uniquely adapted to life with the sea ice, such as polar bears (Ursus maritimus), sea ice loss is expected to cause distributional shifts, declines in body condition, and reduced reproduction rates and populations (Wiig et al. 2008; Kovacs et al. 2011; Bromaghin et al. 2015; Laidre et al. 2015a).
Polar bears occupy annual and multi-year sea ice habitats over continental shelves throughout the Arctic where their primary prey species, ringed seals (Pusa hispida), are found (Stirling and Archibald 1977; Smith 1980). Sea ice serves as a hunting platform from which polar bears find prey, as well as a migration corridor (Stirling 2009). Widespread loss of sea ice habitats has led to negative impacts for polar bears, including reduced body size and condition (Rode et al. 2010, 2012, 2014); decreased survival among juvenile, subadult, adult females, and senescent bears (Regehr et al. 2007, 2010; Bromaghin et al. 2015); shifts in habitat use from annual ice to areas with lower ice concentrations and/or multiyear ice (Laidre et al. 2015b); and increased use of terrestrial habitats (Fischbach et al. 2007; Schliebe et al. 2008; Rode et al. 2015; Olson et al. 2017).
The impact of climate change on polar bear reproductive behavior, specifically maternity den habitat selection and phenology, is poorly understood. Like other bear species, polar bears give birth in maternity dens during the winter months (Harington 1968; Blix and Lentfer 1979). Female polar bears normally reproduce every 2–3 years and mate during the spring season (Lønø 1970; DeMaster and Stirling 1981; Ramsay and Stirling 1988). In autumn, pregnant females excavate dens where they will give birth to 1–3 altricial cubs between December and January (Blix and Lentfer 1979; Ramsay and Stirling 1988; Wiig 1998; Ferguson et al. 2000). After they emerge from the den sometime in March–April, the cubs will remain with their mother for around two and a half years before embarking on their own (Ramsay and Stirling 1988).
While only pregnant females over-winter in dens, bears of other age, sex, and reproductive classes may take shelter in dens during periods of extreme temperatures, to escape inclement weather, or to conserve energy when food is unavailable (Harington 1968; Jonkel et al. 1972; Schweinsburg 1979; Derocher and Stirling 1990; Ramsay et al. 1991; Messier et al. 1994; Ferguson et al. 2000). Bears typically occupy shelter dens between 14 and 20 days (Messier et al. 1994; Born et al. 1997), whereas mother polar bears will remain in maternity dens between 90 and 197 days (Born et al. 1997; Wiig 1998; Ferguson et al. 2000).
Some have speculated that a decrease in sea ice will lead to a change in polar bear denning phenology (e.g., Wiig et al. 2008). However, previous studies examining polar bear denning phenology and habitat are few and focus on only some of the 19 polar bear subpopulations, including Southern Beaufort Sea (Fischbach et al. 2007; Olson et al. 2017), Western Hudson Bay (Ramsay and Stirling 1990; Richardson et al. 2005), East Greenland (Laidre et al. 2015b), McClure Strait and Viscount Melville Sound (Messier et al. 1994), and the Barents Sea (Derocher et al. 2011). The connection between habitat changes and polar bear reproduction in Baffin Bay (BB) and Kane Basin (KB), however, has not been studied.
The present study utilized satellite telemetry data collected on the BB and KB subpopulations from 1991 to 1997 and those from 2009 to 2015 to determine if any shifts in polar bear maternity den phenology and habitat characteristics have occurred over space and time. We hypothesized that in comparison with the 1990s data, pregnant females in the 2000s would leave their dens earlier over time in correlation to the earlier break-up of sea ice. It was also expected that den site characteristics would remain the same over time in accordance with the results of previous studies (Messier et al. 1994; Ferguson et al. 2000).
Methods
Study area
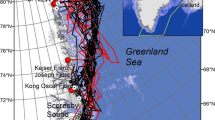
Our study area spanned roughly 1.28 × 106 km2 from 65°N to 82°N and from 50°W to 85°W, including Baffin Bay to the south and the Kane Basin region to the north (Fig. 1). Baffin Bay is a deep-water bay framed by Greenland to the east and Baffin Island to the west. The bay is characterized by a seasonal ice cycle where sea ice almost completely disappears by late summer and begins to refreeze starting in October, followed by a period of complete cover from December to April (Tang et al. 2004). In spring, warm water flowing northward in the West Greenland Current melts sea ice along the western edge of Greenland in a counterclockwise pattern (Tang et al. 2004), with the last ice melting along the coast of Baffin Island (Ferguson et al. 1997). The height of the coastal mountains on islands surrounding Baffin Bay, including Baffin Island, has a strong effect on precipitation patterns in the region with peak precipitation occurring in October (Seidel 1987). Due to its high-elevation mountainous terrain (>2000 m; Ferguson et al. 1997), the eastern coast of Baffin Island receives more precipitation in the form of snow than surrounding islands (Andrews and Barry 1972; Crowe 1976; Maxwell 1980). Average snowfall on the east coast of Baffin Island ranges from 1.3 m year−1 in the north (~72.7°N) and 2.3 m year−1 in the south (~63.7°N; 1981–2010 Climate Normals & Averages, Meteorological Service of Canada 2017). Baffin Bay itself, however, receives very little precipitation (0.1 m year−1; Appenzeller et al. 1998).
Kane Basin lies to the north of Baffin Bay between Ellesmere Island and Greenland. Smith Sound and the North Water Polynya border the region to the south; and the Kennedy Channel borders the region to the north (Fig. 1). Sea ice patterns in the Nares Strait–Kane Basin region are heavily influenced by the influx of multi-year pack ice flowing south from the Arctic Ocean and locally formed ice (Tang et al. 2004; Preußer et al. 2015). The coasts of the islands bordering Nares Strait–Kane Basin, including Ellesmere, Coburg, and Devon Islands (Fig. 1), receive relatively heavy precipitation in the winter (>0.3 m year−1; Ingram et al. 2002).
The Baffin Bay (BB) polar bear subpopulation is contained within the borders created by the North Water Polynya to the north; the coast of Greenland to the east; Baffin Island, Nunavut, Canada, to the west; and Cape Dyer, Baffin Island, and Sisimiut, west Greenland, to the south (Taylor et al. 2001, 2005; Fig. 1). The Kane Basin (KB) subpopulation is contained within the boundary set by the North Water Polynya to the south; Greenland to the east; Ellesmere Island, Canada, to the west; and the Kennedy Channel to the north (Taylor et al. 2001, 2008; PBSG 2010; Fig. 1).
Although polar bears in BB and KB are genetically similar (Paetkau et al. 1999), they are considered two separate subpopulations based on movement studies (Taylor et al. 2001; SWG 2016). The two subpopulations occupy areas with markedly different sea ice regimes. Baffin Bay forms part of the seasonal ice ecoregion, characterized by the formation and near-complete melting of sea ice each summer (Amstrup et al. 2008). In contrast, the Nares Strait–Kane Basin area is incorporated into the archipelago ecoregion, historically characterized by year-round sea ice habitat (Amstrup et al. 2008; Hamilton et al. 2014). However, in the recent decades, sea ice conditions in the Kane Basin region have become more variable and seasonal in nature (Hamilton et al. 2014; Stern and Laidre 2016).
Data collection
Satellite telemetry data were collected from 40 adult BB females from 2009 to 2015, and from 16 adult KB females from 2012 to 2015 (SWG 2016). BB bears were captured in northwest Greenland in the months of April and May of 2009–2013. KB bears were tagged along eastern Ellesmere Island and in the Nares Strait–Kane Basin region between April and May of 2012–2013. All bears were darted and immobilized using methods established by Stirling et al. (1989), and equipped with Telonics TAW–4610H satellite telemetry collars (Telonics, Mesa, AZ). Collars transmitted temperature and activity data during a 6-h period, while position coordinates were transmitted for an 8-h period every 4 days. Temperature was measured by a thermistor within the collar, and activity level was quantified as the count of state changes of a mercury tip-switch sensor (Fischbach et al. 2007). Although the temperature readings are influenced by the animal’s body temperature, they are accepted as a general representation of the ambient temperature of the surrounding habitat (Harris et al. 1990).
We extracted temperature data from downloaded Argos files using the Telonics Data Converter software (Version 2.21; Telonics, Mesa, AZ). The Argos Data Collection and Location System (Toulouse, France) received all transmissions from the collars and assigned a location-quality score (LQ) based on an error radius calculated using the frequency and strength of messages received by the satellites. Positions with an LQ of 0, 1, 2, and 3 are within >1500, 500–1500, 250–500, and <250 m of the true position of the animal, respectively (CLS Argos User Manual 2016). LQ scores of A, B, and Z are of unknown or poor quality. Positions with LQ scores of 1–3 were primarily used for this study, although some entry/exit dates had to be determined using poorer-quality positions due to gaps between best-quality positions.
Argos files for each month were filtered by a speed–distance–angle filter from the “Argosfilter” package developed by Freitas et al. (2008) in the statistical software program R.3.0.2 (R Core Team 2013). The filter calculates the distance between two successive positions and determines whether this distance exceeds the maximum sustainable velocity for a polar bear, which we set as 10 km h−1 (Laidre et al. 2012). Any points inconsistent with the plausible upper travel speed limit were removed from the database.
The 1990s den data were determined by Ferguson et al. (1997, 2000) using satellite telemetry collected from adult females in Baffin Bay (n = 42 bears) and Kane Basin (n = 10 bears) from 1991 to 1997. The 1990s datasets included 29 dens from BB and nine from KB, and the data consisted of one position per day along with the coordinates of the den sites. We did not have access to the raw 1990s data and thus were unable to apply the same filtering method to both datasets.
Determining den locations for the 2000s dens
Solitary females or females with two-year-old cubs are typically considered candidates for denning the following winter after capture (Wiig 1998). However, we chose to examine the location and temperature data for all adult females, including those with cubs-of-the-year or yearlings, from July to June of each year the collars actively transmitted. The activity data provided by the collars were not used in detecting denning behavior since it was difficult to discern between periods of high and low activity during the denning period. Only one position, selected by choosing the first position with the best LQ score, and the daily average temperature were used for each day. We removed temperature readings ≥40 °C or ≤–40 °C which were considered outliers before calculating the average daily temperature (Tchernova 2010). Three variables signal that a female is in a den: high temperature readings compared to ambient air temperature (10–40 °C warmer), constant position on land, and decreased quality and frequency of transmissions (Amstrup and Gardner 1994; Messier et al. 1994; Born et al. 1997; Wiig 1998; Fischbach et al. 2007). Temperature data were available for all BB bears; however, only four of the KB bears provided useable temperature data.
Position data were particularly coarse as points within the denning period did not closely center on a single den position. For their study, Andersen et al. (2012) used the first best-quality position after emergence as the den position. However, since the collars for the present study transmitted for only a 6- to 8-h period every 4 days, an instrumented bear could have traveled some distance away from the actual den site before providing a high-quality position. Instead, we devised a method to determine the den site positions for the 2000s bears. First, we inspected the data for all adult females for sustained high temperatures (>0 °C), decreased transmission quality and frequency, and a stationary position on land during the denning period (September–March). Since females give birth in mid-November to mid-January (Harington 1968; Derocher et al. 1992), they should be in their dens from December to February. Therefore, we created a subset of the position data for each denning candidate during this period and mapped the points in ArcMap v.10.1 (Environmental Systems Research Institute, Inc. [ESRI] 2012). The point files were then separated based on their LQ score, after which we created a buffer around each point with radii equivalent to the maximum error estimate for each LQ score (LQ 1 had a buffer of 1500 m, LQ 2: 500 m, LQ 3: 250 m). The mean center of the intersection of these buffers was then determined as the den position. This approach provides a probable location for each den site based on the error estimate of the satellite telemetry positions during the denning period, and is independent of the number of positions as well as spatial outliers.
Not all dens were determined using this method since some of the bears had sparse location data within the denning period, or the buffers did not overlap. Consequently, the den positions in these cases had to be determined using variants of the buffer method (shelter dens n = 5; maternity dens n = 6). Among the five shelter dens, three of the dens only had LQ 1 and LQ 3 buffers, or LQ 2 and LQ 3 buffers; thus, the mean centers of the intersection between the two buffers were used as the den sites. For the other two shelter dens as well as three of the six maternity dens, the three LQ buffers did not overlap, and therefore, the mean centers of the points were used as the den sites. For the remaining three maternity dens, the bears had sparse data during the denning period (December–February), and thus, subsets of the points were used to calculate the mean centers as the den sites.
Maternity den phenology analyses
Duration of den occupation was used to distinguish between bears denning in maternity dens and those denning in shelter dens. Although we quantified the number of shelter dens for each subpopulation, we did not analyze the phenology and habitat characteristics of the shelter dens. In addition to denning duration, we calculated and compared the entry and exit dates for the 2000s maternity dens to the 1990s data (Ferguson et al. 2000). Most entry dates (n = 27) for the 2000s dens were determined by creating a 1-km buffer around each den site and taking the median date between the last transmission outside of the buffer and the first transmission inside the buffer. The entry date was then verified by comparing the autumn temperature data for each denning bear with temperature readings from a non-denning bear that same year to check for a difference of more than 10° C. The dates when the temperature readings diverged by ≥10° C were then compared to the entry dates determined by position data. The exit date for each den was established as the median date between the female’s last transmission from the den and the first movement outside the den, as indicated by a significant drop in temperature and movement outside of a 1-km buffer around the den site. Details on determination of entry and exit dates for all dens can be found in Appendix I of Escajeda (2016). Den entry and exit dates were measured as day-of-year (DOY day #1 is 1 January), which we then used to calculate denning duration in number of days (Messier et al. 1994; Born et al. 1997; Wiig 1998; Ferguson et al. 2000).
We applied two-sample Mann–Whitney U tests to the maternity den temporal data from the two subpopulations to test for differences between the entry/exit dates and denning duration between the 1990s and 2000s datasets without assuming normality. In addition, we calculated a Kendall rank-correlation coefficient to test for correlation between den phenology and latitude. An alpha value of 0.05 was used for all statistical analyses.
First date on land
In addition to analyzing den phenology, the date of entry onto land was determined for each pregnant female. First date on land (FDOL) was defined as the date that the bear first came onto land without returning to the sea ice until she emerged from her den in spring. In the 2000s datasets, some (n = 5) of the FDOLs had to be determined using poorer-quality positions (LQ of 0, A, B, and Z) due to gaps between best-quality positions. For seven of the 1990s maternity dens, the date that the bears first came onto land was also the listed den entry date. Two BB bears in the 2000s dataset returned to the sea ice before entering their den, and thus had two FDOLs: (1) the first date they came onto land in the late summer/early autumn, and (2) the date they came back on land before entering their dens. Two-sample Mann–Whitney U tests were applied to two data matrices: BB bears with first FDOL for the two bears, and BB bears with the second FDOL.
Den habitat characteristics analyses
All den positions were imported into ArcMap and overlaid with a digital elevation model (DEM) of the study area. The DEM consisted of a mosaic of tiles from the Advanced Spaceborne Thermal Emission and Reflection Radiometer Global Digital Elevation Model (ASTER GDEM Version 2), a product of Japan’s Ministry of Economy, Trade, and Industry (METI) and the United States National Aeronautics and Space Administration (NASA). The ASTER GDEM had an overall vertical accuracy of 17 m at the 95% confidence level. The DEM was produced at a 22.625 × 22.625 m resolution in the WGS 1984 datum and projected in a North Pole Stereographic projection with a central meridian of –55°W. The elevation, aspect, and slope of each den site were extracted from the DEM, while straight-line distance to the nearest shoreline was measured using a vector coastline of Canada (US Defense Mapping Agency). Aspect gives the direction of the maximum rate of downslope change from a cell to its neighbors (in degrees), while slope measures the rate of maximum change of elevation (in degrees). Two-sample Mann–Whitney U tests were used to compare elevation, slope, and distance to coast of maternity dens between the 1990s and 2000s datasets. Den aspects were tested for uniform circular distribution using a Rayleigh’s test, and the two datasets were compared using a nonparametric two-sample Watson’s U 2 Test for circular data. We categorized aspect as north = >337.5°–22.5°; northeast = >22.5°–67.5°; east = >67.5°–112.5°; southeast = >112.5°–157.5°; south = >157.5°–202.5°; southwest = >202.5°–247.5°; west = >247.5°–292.5°; and northwest = >292.5°–337.5°.
Results
Baffin Bay subpopulation
Our analysis identified 21 BB dens between 2009 and 2015, including 16 maternity dens (Fig. 1) and five shelter dens (Fig. 2). Ferguson et al. (1997) found 29 dens from BB in 1991–1997 with eight maternity dens and 21 shelter dens. All but one of the dens from the BB subpopulation were located on land (one 1990s shelter den was located on landfast ice inside a fjord near the shore of Baffin Island), and the majority were found on Baffin Island (n = 38). Exceptions included four dens located on Bylot Island, two dens on Coburg Island, one den on Devon Island, one den on Prince Wales Island, and two dens in the Melville Bay region of northwest Greenland (maternity dens Fig. 1; shelter dens Fig. 2). The maternity den on Greenland was included in the phenology analyses but not the habitat characteristics analyses since we did not have any dens in Greenland in the 1990s datasets for comparison. One BB bear denned a little less than 35 km away from Qikiqtarjuaq, Baffin Island, Canada, while most bears denned far from human settlements (\(\bar{x}\) = 143.1 km).
There were a few interesting cases to note from the BB subpopulation. One bear used a den in 2012 that was ~1.25 km away from her previous denning site from 2009 on a peninsula close to Eglinton Fjord, Baffin Island. This was the only case where a bear exhibited fidelity to a denning area among the bears that built a maternity den twice in our dataset (n = 3). The other females denned in areas far from previous den sites (>300 km). Also, another female constructed two maternity dens in consecutive years (2011 and 2012), indicating that she either lost her cubs, or her pregnancy failed.
Kane Basin subpopulation
For the KB subpopulation, nine dens were identified from 2012 to 2015, consisting of three maternity dens (Fig. 1) and six shelter dens (Fig. 2). Ferguson et al. (1997) identified nine dens total with three maternity dens and six shelter dens from 1992 to 1995. Almost all dens were on land, except for one 1990s shelter den that was located on landfast ice ~9 km from the shore of Ellesmere Island. Most dens were located on Ellesmere Island except for three shelter dens on Devon Island (Figs. 1, 2). No females from the KB subpopulation denned on Greenland.
Maternity den phenology
In the BB subpopulation, female polar bears in the 2000s on average spent 27 fewer days in their maternity dens (\(\bar{x}\) = 167.1 days, SD = 27.6 days, n = 15) than in the 1990s (\(\bar{x}\) = 194.1 days, SD = 21 days, n = 8; Mann–Whitney U = 23.5, p = 0.017; Table 1). Timings of den entry differed significantly among the two periods (Mann–Whitney U = 96, p = 0.018); however, we did not find a significant difference among exit dates (Mann–Whitney U = 73.5, p = 0.399). The median entry date for the 2000s dens (3 October) was more than a full month later than the median date of entry for dens in the 1990s (29 August). Therefore, differences in entry dates accounted for the observed difference in duration among the two time periods. There was no significant correlation between latitude and den entry (τ = 0.135, p = 0.383), exit date (τ = 0.194, p = 0.212), or den duration (τ = −0.167, p = 0.278) for the BB maternity dens.
For the KB subpopulation, the sample sizes were too small to conduct statistical tests for differences in den duration, entry or exit dates between the two time periods (Table 1). We were also unable to test for correlation between latitude and maternity den phenology.
First date on land
Most of the bears came onto land and then slowly made their way to the den area. BB bears averaged 9 days from the FDOL to the den entry date in the 1990s compared to 58 days in the 2000s. KB bears had a narrower gap with an average of 20 days between the FDOL and den entry date in the 1990s and 32 days in the 2000s. With the first FDOL for the two BB bears, the overall FDOL for BB maternity dens advanced from a median DOY of 237 (SD = 19, n = 8) in the 1990s to a median DOY of 219 (SD = 9.1, n = 16; Mann–Whitney U = 112, p = 0.002; Fig. 3) in the 2000s. The second FDOL matrix produced similar results; however, the median for the 2000s dens was later at a DOY of 221 (Mann–Whitney U = 100.5, p = 0.024). For KB maternity dens, FDOL advanced from a median DOY of 261 (SD = 31, n = 3) in the 1990s to a median DOY of 235 (SD = 20.8, n = 3; Fig. 3).
Den habitat characteristics
For BB dens, elevation increased from a mean of 351.3 m (SD = 194.5 m, n = 8) in the 1990s to 707.0 m (SD = 284.9 m, n = 15; Mann–Whitney U = 105, p = 0.003; Fig. 4) in the 2000s, while slope increased from a mean of 11.9° (SD = 6.4°) in the 1990s to 23.1° (SD = 7.4°) in the 2000s (Mann–Whitney U = 105, p = 0.003; Fig. 4). Most BB maternity dens had southerly aspects in the 2000s (\(\bar{x}\) = 199.7°, r = 0.47, n = 15), whereas in the 1990s, dens with northerly aspects were more common: (\(\bar{x}\) = 51.3°, r = 0.58, n = 8; Watson’s U 2 = 0.191, p = 0.042; Fig. 4). The 2000s BB maternity den aspects were not uniformly distributed (Rayleigh’s test p = 0.041) and thus had a significant sample mean direction. In contrast, the 1990s BB maternity dens were found to have a slight uniform circular distribution (Rayleigh’s test p = 0.065). Females in both subpopulations maintained similar distance to coast between the 1990s and 2000s (BB dens Mann–Whitney U = 55, p = 0.776; Table 2; Figs. 4, 5).
Plots comparing the aspect, slope, elevation, and distance to coast of the 1990s (n = 8) and 2000s (n = 15) polar bear maternity dens in Baffin Bay (Greenland den was omitted). The aspect plot consists of a simplified circular histogram with the cardinal and ordinal directions that dens faced. The length of each line is proportional to the frequency of dens at the same aspect. Elevation (p = 0.003), slope (p = 0.003), and aspect (p = 0.042) significantly differed between the two time periods, whereas no significant difference was detected for distance to coast (p = 0.776)
Plots comparing the aspect, slope, elevation, and distance to coast of the 1990s (n = 3) and 2000s (n = 3) polar bear maternity dens in Kane Basin. The aspect plot consists of a simplified circular histogram with the cardinal and ordinal directions dens faced. The length of each line is proportional to the frequency of dens at the same aspect
Sample sizes limited the comparison of habitat characteristics among KB dens; however, our results suggest no significant differences for the two time periods, with similar elevations (1990s \(\bar{x}\) = 207.7 m, SD = 206.9 m, n = 3; 2000s \(\bar{x}\) = 446.3 m, SD = 56.4 m, n = 3; Fig. 5) and slope (1990s \(\bar{x}\) = 11.9°, SD = 7.1°, n = 3; 2000s \(\bar{x}\) = 15°, SD = 3.8°, n = 3; Fig. 5). The majority of KB maternity dens had southerly aspects (1990s \(\bar{x}\) = 200.7°, r = 0.94, n = 3; 2000s \(\bar{x}\) = 159.7°, r = 0.39, n = 3; Fig. 5).
Discussion
The purpose of this study was to determine whether the phenology and habitat characteristics of polar bear maternity dens have shifted in BB and KB since the 1990s. In BB, maternity den duration in the 2000s was significantly shorter than the duration in the 1990s due to females entering their dens later in the autumn. In addition, compared to BB maternity dens in the 1990s, dens in the 2000s were found at higher elevations and steeper slopes. Den aspect also varied between the two time periods, with more dens at southerly aspects in the 2000s than those in the 1990s. BB adult females, whether pregnant or not, also came onto land significantly earlier in the year in the 2000s. We were unable to detect any shifts in KB maternity denning patterns due to small sample sizes (Table 2).
There are a limited number of studies examining polar bear maternity denning phenology and, like this study, only focus on a subset of polar bear subpopulations. For the East Greenland subpopulation, Laidre et al. (2015b) compared maternity dens during 2007–2010 to den data from 1993 to 1998 published by Born et al. (1997) and Wiig et al. (2003). Even though this region has experienced significant ice loss since the 1990s, Laidre et al. (2015b) found that females entered their dens earlier (\(\bar{x}\) = 6 October; p = 0.064) and had longer denning duration (\(\bar{x}\) = 174 days; p = 0.097) in 2007–2010 than in 1993–1998 (entry date \(\bar{x}\) = 26 October; duration \(\bar{x}\) = 158 days). Exit dates did not differ between the two periods (1993–1998 \(\bar{x}\) = 1 April; 2007–2010 \(\bar{x}\) = 29 March; p = 0.609; Laidre et al. 2015b). According to a study by Wiig (1998) on Barents Sea females denning in Svalbard, Norway, from 1988 to 1993, the average entry date was 7 November, and duration lasted on average 153 days. As for exit dates among the Barents Sea subpopulation, Wiig (1998) reported an average exit date of 5 April, whereas Andersen et al. (2012) observed females leaving their dens between 29 March and 27 April (\(\bar{x}\) = 17 April) from 1972 to 2010. Using satellite telemetry, Messier et al. (1994) found that bears in the Viscount Melville Sound subpopulation entered their dens earlier during 1989–1992 (\(\bar{x}\) = 17 September) in comparison to qualitative data on polar bears in the High Arctic (Harington 1968; Jonkel et al. 1972; Stirling et al. 1980). Exit dates in Messier et al. (1994) were about the same. Harington (1968) directly observed females entering their dens on eastern Baffin Island from 1–7 October, considerably later than the range for the 1990s dens published by Ferguson et al. (2000) and reported here (1990s entry date \(\bar{x}\) = 7 September; 2000s entry date \(\bar{x}\) = 5 October); however, his methods did not utilize satellite telemetry. From these studies, it is clear that den entry dates and duration vary among subpopulations, whereas females leave their dens around the same time frame (March–April). More research is therefore needed to further elucidate the extent of plasticity in denning phenology in response to changing habitat conditions in polar bear subpopulations occupying areas with different sea ice regimes.
Previous works examining shifts in den distribution are similarly restricted to a small portion of the polar bear range, and most of them examined shifts in den distribution in relation to changes in sea ice (Southern Beaufort Sea: Fischbach et al. 2007; Olson et al. 2017; Barents Sea: Derocher et al. 2011). Continued monitoring of changes in den phenology and habitat in BB, KB, and other subpopulations is needed.
There have been extensive changes in the BB region in the past few decades. Break-up of sea ice has advanced, forcing bears on shore ~30 days earlier in the summer (SWG 2016). In BB, the sea ice melted an average of 7 days per decade earlier in the spring and formed an average 5.2 days per decade later in the autumn from 1979 to 2013, lengthening the ice-free period (Laidre et al. 2015a). Reduced access to sea ice has been to shown to negatively affect the mass of potentially pregnant females in other subpopulations (Stirling and Parkinson 2006; Regehr et al. 2010). The body fat of a pregnant female determines her ability to successfully reproduce, and her mass is directly correlated with the mass of her cubs at emergence (Derocher and Stirling 1996, 1998; Derocher et al. 2011). If females arrive on Baffin Island in poor condition, they may require more time hunting in remnant coastal fast ice, or remains of the pack ice, to build up fat reserves before denning. The longer period between arrival on land and den entry among the BB bears in the 2000s dataset supports this hypothesis (58 days in the 2000s compared to 9 days in the 1990s).
Also, two BB females in the 2000s dataset returned to the sea ice in the late autumn after they arrived on Baffin Island. However, entry dates from these two females did not affect the statistical significance of the results (den entry p with these bears removed = 0.042). Previous studies have documented pregnant females hunting out on the sea ice as late as December (Koettlitz 1898; Harington 1962), as well as bears leaving their dens to return to the coast to hunt (Harington 1964). With less time to hunt out on the sea ice in BB, bears are coming onto land in poorer condition (Rode et al. 2012; SWG 2016). Thus, pregnant females in BB may be under food stress due to early sea ice break-up and, consequently, may try to maximize their time hunting and scavenging rather than immediately establishing a den site.
Alternatively, as the sea ice melts earlier and snowfall is reduced, bears may have to travel farther to access preferred habitat (Derocher et al. 2004). For pregnant females, this means traveling greater distances to find favorable denning locations. In general, female polar bears do not exhibit fidelity to specific den sites, but some have shown fidelity to general denning areas (Ramsay and Stirling 1990; Amstrup and Gardner 1994; Zeyl et al. 2010). A female may select a den site known to have a relatively short route back to desirable hunting grounds once she emerges in the spring (Ramsay and Andriashek 1986; Ramsay and Stirling 1990). By this reasoning, if a female is on land far from optimal habitat, she may choose to travel to more favorable denning regions even if it means establishing her den later in the year.
The most congruent explanation for the average 28-day delay in BB entry dates and the observed increases in elevation and slope is that there are fewer multi-year snowdrifts at lower elevations due to warmer temperatures. Females in 2009–2015 likely climbed to higher elevations to find suitable snow accumulations in which to excavate dens of suitable size (Liston et al. 2016). Thus, the presence of snowdrifts is most likely an important factor in den selection for BB females. Examining average snow depth as well as the distribution of snowdrifts in maternity denning regions should be explored in future studies.
In addition to higher elevations and steeper slopes, BB dens in the 2000s were primarily south-facing (n = 9 out of 15) in contrast to the 1990s when there were more north-facing dens (n = 5 out of 8). In the Canadian Arctic Archipelago, south-facing mountain slopes tend to have more snow due to precipitation transport by northerly winds (Harington 1968; Schweinsburg et al. 1984). They also receive more solar radiation in the spring, creating a warmer environment for the cubs when they emerge (Van de Velde 1957; Harington 1962). It is possible that in the 1990s, females were able to select snow drifts retained from previous winters on north-facing aspects. In the 2000s, these snowdrifts may have been less available, and consequently, females selected south-facing aspects that provided the earliest snow accumulations from early-winter storms. More recent observations on the relationship between aspect, and snowdrift deposition and retention are needed to support this hypothesis.
Using satellite telemetry data, we developed a procedure for modeling den positions and calculating entry and exit dates using the polar bears’ movement and temperature data. Since we were unable to ground-truth our methods, the phenology results and den positions for the 2000s dens are approximations. In addition, because the raw 1990s data were not available, we could not independently determine the 1990s den positions or entry/exit dates. Nonetheless, the difference in BB denning phenology, elevation, slope, and aspect between the 2000s and 1990s is remarkable and statistically significant.
Phenological studies require long term monitoring and years of observations in order to connect phenology shifts to environmental modifications brought on by climate change. In general, a minimum of ~20 years of continuous data is recommended for studies examining the biological impact of climate change (Rosenzweig et al. 2008; Brown et al. 2016). Our data span a period of substantial environmental change in Baffin Bay and thus offer a view of how polar bear denning behavior may be responding to these changes. Of note, we did not monitor the subpopulations between 1997 and 2009, thus creating a gap in the time series. To confidently ascribe the shift in den entry dates to climate change, more data over a longer time span are needed. Rapid changes occurring in the Arctic due to climate warming, however, leave little doubt that a lack of adequate denning substrate in the form of snowdrifts and early sea ice break-up may be affecting maternity denning in BB. The results of this study highlight the need for monitoring changes in snowpack and sea ice throughout the Arctic, and for better understanding of their impact on maternity den phenology and habitat selection.
References
Amstrup SC, Gardner C (1994) Polar bear maternity denning in the Beaufort Sea. J Wildl Manage 58:1–10
Amstrup SC, Marcot BG, Douglas DC (2008) A Bayesian network approach to forecasting the 21st century worldwide status of polar bears. In: DeWeaver ET, Bitz CM, Tremblay LB (eds) Arctic sea ice decline: observations, projections, mechanisms, and implications, vol 180. Geophysical Monograph Series, Washington DC, pp 213–268
Andersen M, Derocher AE, Wiig Ø, Aars J (2012) Polar bear (Ursus maritimus) maternity den distribution in Svalbard, Norway. Polar Biol 35:499–508
Andrewartha HG, Birch LC (1954) The distribution and abundance of animals. University of Chicago Press, Chicago
Andrews JT, Barry RG (1972) Present and paleo-climatic influences on the glacierization and deglacierization of Cumberland Peninsula, Baffin Island, N.W.T., Canada. INSTAAR Occasional Paper Number 2, University of Colorado Institute of Arctic and Alpine Research, Boulder, Colorado
Appenzeller C, Schwander J, Sommer S, Stocker TF (1998) The North Atlantic Oscillation and its imprint on precipitation and ice accumulation in Greenland. Geophys Res Lett 25:1939–1942
Badeck FW, Bondeau A, Böttcher K, Doktor D, Lucht W, Schaber J, Sitch S (2004) Responses of spring phenology to climate change. New Phytol 162:295–309
Blix AS, Lentfer JW (1979) Modes of thermal protection in polar bear cubs—at birth and on emergence from the den. Am J Physiol 236:R67–R74
Born EW, Wiig Ø, Thomassen J (1997) Seasonal and annual movements of radio-collared polar bears (Ursus maritimus) in Northeast Greenland. J Mar Syst 10:67–77
Bromaghin JF, McDonald TL, Stirling I, Derocher AE, Richardson ES, Regehr EV, Douglas DC, Durner GM, Atwood T, Amstrup SC (2015) Polar bear population dynamics in the southern Beaufort Sea during a period of sea ice decline. Ecol Appl 25:634–651
Brown CJ, O’Connor MI, Poloczanska ES, Schoeman DS, Buckley LB, Burrows MT, Duarte CM, Halpern BS, Pandolfi JM, Parmesan C, Richardson AJ (2016) Ecological and methodological drivers of species’ distribution and phenology responses to climate change. Glob Change Biol 22:1548–1560
CLS (Collecte Localisation Satellites) (2016) Argos user’s manual. http://www.argos-system.org/manual/. Accessed 16 March 2016
Crowe RB (1976) A climate classification of the Northwest Territories for recreation and tourism. Environment Canada, Toronto
DeMaster DP, Stirling I (1981) Ursus maritimus. Mamm Species 145:1–7
Derocher AE, Stirling I (1990) Distribution of polar bears (Ursus maritimus) during the ice-free period in western Hudson Bay. Can J Zool 68:1395–1403
Derocher AE, Stirling I (1996) Aspects of survival in juvenile polar bears. Can J Zool 74:1246–1252
Derocher AE, Stirling I (1998) Maternal investment and factors affecting offspring size in polar bears (Ursus maritimus). J Zool 245:253–260
Derocher AE, Stirling I, Andriashek D (1992) Pregnancy rates and serum progesterone levels of polar bears in western Hudson Bay. Can J Zool 70:561–566
Derocher AE, Lunn NJ, Stirling I (2004) Polar bears in a warming climate. Integr Comp Biol 44:163–176
Derocher AE, Andersen M, Wiig Ø, Aars J, Hansen E, Biuw M (2011) Sea ice and polar bear den ecology at Hopen Island, Svalbard. MEPS 441:273–279
Escajeda ED (2016) Identifying shifts in maternity den phenology and habitat characteristics of polar bears (Ursus maritimus) in Baffin Bay and Kane Basin. Master’s Thesis, University of Washington, Washington
ESRI (2012) ArcGIS desktop: release 10. Environmental Systems Research Institute, Redlands
Ferguson SH, Taylor MK, Messier F (1997) Space use by polar bears in and around Auyuittuq National Park, Northwest Territories, during the ice-free period. Can J Zool 75:1585–1594
Ferguson SH, Taylor MK, Rosing-Asvid A, Born EW, Messier F (2000) Relationships between denning of polar bears and conditions of sea ice. J Mammal 81:1118–1127
Fischbach AS, Amstrup SC, Douglas DC (2007) Landward and eastward shift of Alaskan polar bear denning associated with recent sea ice changes. Polar Biol 30:1395–1405
Freitas C, Lydersen C, Fedak MA, Kovacs KM (2008) A simple new algorithm to filter marine mammal Argos locations. Mar Mammal Sci 24:315–325
Hamilton SG, Castro de la Guardia L, Derocher AE, Sahanatien V, Tremblay B, Huard D (2014) Projected polar bear sea ice habitat in the Canadian Arctic Archipelago. PLoS ONE 9:e113746
Harington CR (1962) Polar bear study—Resolute Bay area, Northwest Territories, 1961. Can Wild Serv Rep
Harington CR (1964) Field notes. Southampton Island, Northwest Territories, 1964
Harington CR (1968) Denning habits of the polar bear (Ursus maritimus Phipps). Can Wild Serv Rpt Series 5
Harris RB, Fancy SG, Douglas DC, Garner GW, Amstrup SC, McCabe TR, Pank LF (1990) Tracking wildlife by satellite: current systems and performance. Falls Church, U.S. Fish and Wildl Serv Tech Rep, p 30
Ingram RG, Bâcle J, Barber DG, Gratton Y, Melling H (2002) An overview of physical processes in the North Water. Deep-Sea Res Pt II 49:4893–4906
IPCC (2014) Climate change 2014: synthesis report contribution of working groups I, II, and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In: Core Writing Team, Pachauri RK, Meyer LA (eds) IPCC, Geneva
Jonkel CJ, Kolenosky GB, Robertson RJ, Russell RH (1972) Further notes on polar bear denning habits. Int Conf Bear Biol Manage 2:142–158
Koettlitz R (1898) Contributions to the natural history of the polar bear (Ursus maritimus, Linn.). Proc R Phys Soc 14:78–112
Kovacs KM, Lydersen C, Overland JE, Moore SE (2011) Impacts of changing sea-ice conditions on Arctic marine mammals. Mar Biodiv 41:181–194
Laidre KL, Stirling I, Lowry LF, Wiig Ø, Heide-Jørgensen MP, Ferguson SF (2008) Quantifying the sensitivity of arctic marine mammals to climate-induced habitat change. Ecol Appl 18:S97–S125
Laidre KL, Born EW, Gurarie E, Wiig Ø, Dietz R, Stern H (2012) Females roam while males patrol: divergence in breeding season movements of pack-ice polar bears (Ursus maritimus). Proc R Soc B 280:20122371
Laidre KL, Stern H, Kovacs KM, Lowry L, Moore SE, Regehr EV, Ferguson SH, Wiig Ø, Boveng P, Angliss RP, Born EW, Litovka D, Quakenbush L, Lydersen C, Vongraven D, Ugarte F (2015a) Arctic marine mammal population status, sea ice habitat loss, and conservation recommendations for the 21st century. Conserv Biol 29:724–737
Laidre KL, Born EW, Heagerty P, Wiig Ø, Stern H, Dietz R, Aars J, Andersen M (2015b) Shifts in female polar bear (Ursus maritimus) habitat use in East Greenland. Polar Biol 38:879–893
Liston GE, Perham CJ, Shideler RT, Cheuvront AN (2016) Modeling snowdrift habitat for polar bear dens. Ecol Model 320:114–134
Lønø O (1970) The polar bear (Ursus maritimus Phipps) in the Svalbard area. Nor Polarinst Skr 149:1–115
Maxwell JB (1980) The climate of the Canadian Arctic islands and adjacent waters. Atmospheric Environment Service, Toronto
Messier F, Taylor MK, Ramsay MA (1994) Denning ecology of polar bears in the Canadian Arctic archipelago. J Mammal 75:420–430
Meteorological Service of Canada, Environment Canada (2017) 1981–2010 Climate Normals & Averages. http://climate.weather.gc.ca/climate_normals/index_e.html. Accessed 11 May 2017
Olson JW, Rode KD, Eggett D, Smith TS et al (2017) Collar temperature sensor data reveal long-term patterns in southern Beaufort Sea polar bear den distribution on pack ice and land. MEPS 564:211–224
Paetkau D, Amstrup SC, Born EW et al (1999) Genetic structure of the world’s polar bear populations. Mol Ecol 8:1571–1584
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42
Parry ML, Canziani OF, Palutikof JP, van der Linden PJ, Hanson CE (eds) (2007) Contribution of working group II to the fourth assessment report of the intergovernmental panel on climate change, 2007. Cambridge University Press, Cambridge
PBSG (Polar Bear Specialist Group) (2010) In: Obbard ME, Thiemann GW, Peacock E, DeBruyn T (eds) Proceedings of 15th Working Meeting of the Polar Bear Specialist Group IUCN, Copenhagen, Denmark. Occasional Papers of the IUCN Species Survival Commission No. 43. Gland and Cambridge
Peters RL (1992) Conservation of biological diversity in the face of climate change. In: Peters RL, Lovejoy TE (eds) Global warming and biological diversity. Yale University, New Haven, pp 15–30
Preußer A, Heinemann G, Willmes S, Paul S (2015) Multi-decadal variability of polynya characteristics and ice production in the North Water Polynya by means of passive microwave and thermal infrared satellite imagery. Remote Sens 7:15844–15867
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0, http://www.R-project.org/
Ramsay MA, Andriashek DS (1986) Long distance route orientation of female polar bears (Ursus maritimus) in spring. J Zool 208:63–72
Ramsay MA, Stirling I (1988) Reproductive biology of female polar bears (Ursus maritimus). J Zool 214:601–634
Ramsay MA, Stirling I (1990) Fidelity of female polar bears to winter-den sites. J Mammal 71:233–236
Ramsay MA, Nelson RA, Stirling I (1991) Seasonal changes in the ration of serum urea to creatinine in feeding and fasting polar bears. Can J Zool 69:298–302
Regehr EV, Lunn NJ, Amstrup SC, Stirling I (2007) Effects of earlier sea ice breakup on survival and population size of polar bears in western Hudson Bay. J Wildl Manage 71:2673–2683
Regehr EV, Hunter CM, Caswell H, Amstrup SC, Stirling I (2010) Survival and breeding of polar bears in the southern Beaufort Sea in relation to sea ice. J Anim Ecol 79:117–127
Richardson E, Stirling I, Hik DS (2005) Polar bear (Ursus maritimus) maternity denning habitat in western Hudson Bay: a bottom-up approach to resource selection functions. Can J Zool 83:860–870
Rode KD, Amstrup SC, Regehr E (2010) Reduced body size and cub recruitment in polar bears associated with sea ice decline. Ecol Appl 20:768–782
Rode KD, Peacock E, Taylor M, Stirling I, Born EW, Laidre KL, Wiig Ø (2012) A tale of two polar bear populations: ice habitat, harvest, and body condition. Popul Ecol 54:3–18
Rode KD, Regehr EV, Douglas DC, Durner G, Derocher AE, Thiemann GW, Budge SM (2014) Variation in the response of an Arctic top predator experiencing habitat loss: feeding and reproductive ecology of two polar bear populations. Glob Change Biol 20:76–88
Rode KD, Wilson RR, Regehr EV, St. Martin M, Douglas DC, Olson J (2015) Increased land use by Chukchi Sea polar bears in relation to changing sea ice conditions. PLoS ONE 10:e0142213
Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA (2003) Fingerprints of global warming on wild animals and plants. Nature 421:57–60
Rosenzweig C, Karoly D, Vicarelli M et al (2008) Attributing physical and biological impacts to anthropogenic climate change. Nature 453:353–357
Schliebe S, Rode KD, Gleason JS, Wilder J, Proffitt K, Evans TJ, Miller S (2008) Effects of sea ice extent and food availability on spatial and temporal distribution of polar bears during the fall open-water period in the Southern Beaufort Sea. Polar Biol 31:999–1010
Schweinsburg RE (1979) Summer snow dens used by polar bears in the Canadian High Arctic. Arctic 32:165–169
Schweinsburg RE, Spencer W, Williams D (1984) Polar bear denning area at Gateshead Island, Northwest Territories. Arctic 37:169–171
Seidel KH (1987) The climate of Auyuittuq National Park Reserve: a review. Government of the Northwest Territories, Yellowknife
Smith TG (1980) Polar bear predation of ringed and bearded seals in the land-fast sea ice habitat. Can J Zool 58:2201–2209
Sparks TH, Menzel A (2002) Observed changes in seasons: an overview. Int J Climatol 22:1715–1725
Stern HL, Laidre KL (2016) Sea-ice indicators of polar bear habitat. Cryosphere 10:2027–2041
Stirling I (2009) Polar bears. In: Perrin WF, Würsig B, Thewissen JGM (eds) Encyclopedia of marine mammals. Academic Press, San Diego, pp 88–890
Stirling I, Archibald WR (1977) Aspects of predation of seals by polar bears. J Fish Res Board Can 34:1126–1129
Stirling I, Parkinson CL (2006) Possible effects of climate warming on selected populations of polar bears (Ursus maritimus) in the Canadian Arctic. Arctic 59:261–275
Stirling I, Calvert W, Andriashek D (1980) Population ecology studies of the polar bear in the area of southeastern Baffin Island. Can Wildl Serv Occas Pap 44:1–33
Stirling I, Spencer C, Andriashek D (1989) Immobilization of polar bears (Ursus maritimus) with Telazol in the Canadian Arctic. J Wildl Dis 25:159–168
SWG (Scientific Working Group to the Canada-Greenland Joint Commission on Polar Bear (2016) Re-Assessment of the Baffin Bay and Kane Basin polar bear subpopulations: Final Report to the Canada-Greenland Joint Commission on Polar Bear, 31 July 2016
Tang CCL, Ross CK, Yao T, Petrie B, DeTracey BM, Dunlap E (2004) The circulation, water masses and sea-ice of Baffin Bay. Prog Oceanogr 63:183–228
Taylor MK, Akeeagok S, Andriashek D, Barbour W, Born EW, Calvert W, Cluff HD, Ferguson S, Laake J, Rosing-Asvid A, Stirling I, Messier F (2001) Delineating Canadian and Greenland polar bear (Ursus maritimus) populations by cluster analysis of movements. Can J Zool 79:690–709
Taylor MK, Laake J, McLoughlin PD, Born EW, Cluff HD, Ferguson SH, Rosing-Asvid A, Schweinsburg R, Messier F (2005) Demography and viability of a hunted population of polar bears. Arctic 58:203–214
Taylor MK, Laake J, McLoughlin PD, Cluff HD, Born EW, Rosing-Asvid A, Messier F (2008) Population parameters and harvest risks for polar bears (Ursus maritimus) of Kane Basin, Canada and Greenland. Polar Biol 31:491–499
Tchernova J (2010) Denning characteristics and movement patterns of female polar bears with cubs in Svalbard during the first month after emergence: implication for detecting denning locations. Master’s Thesis, University of Tromsø, Tromsø
Thackeray SJ, Sparks TH, Frederiksen M et al (2010) Trophic level asynchrony in rates of phenological change for marine, freshwater and terrestrial environments. Glob Change Biol 16:3304–3313
Van de Velde F (1957) Nanuk, king of the arctic beasts. Eskimo 45:4–15
Vaughan DG, Comiso JC, Allison I, et al (2013) Observations: cryosphere. In: Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJ, Fromentin JM, Hoegh-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416:389–395
Wassmann P, Duarte CM, Agustí S, Sejr MK (2011) Footprints of climate change in the Arctic marine ecosystem. Glob Change Biol 17:1235–1249
Wiig Ø (1998) Survival and reproductive rates for polar bears at Svalbard. Ursus 10:25–32
Wiig Ø, Born EW, Pedersen LT (2003) Movements of female polar bears (Ursus maritimus) in the East Greenland pack ice. Polar Biol 26:509–516
Wiig Ø, Aars J, Born E (2008) Effects of climate change on polar bears. Sci Prog 91:151–173
Williams TM, Noren SR, Glenn M (2010) Extreme physiological adaptations as predictors of climate-change sensitivity in the narwhal, Monodon monoceros. Mar Mamm Sci 27:334–349
Zeyl E, Ehrich D, Aars J, Bachmann L, Wiig Ø (2010) Denning-area fidelity and mitochondrial DNA diversity of female polar bears (Ursus maritimus) in the Barents Sea. Can J Zool 88:1139–1148
Acknowledgements
The authors wish to offer their thanks to the Scientific Working Group of the Canada-Greenland Joint Commission on Baffin Bay and Kane Basin Polar Bears for providing the 2000s satellite telemetry data. Thanks are also due to A. Hornof, M. Logsdon, H. Stern, and R. McGovern for their assistance with analyses and GIS, and to the local hunters and technicians from Greenland and Canada for their assistance in the field. Funding for the polar bear fieldwork was provided by the Greenland Institute of Natural Resources, the Government of Greenland; the Danish Ministry of Environment (Miljøstøtte til Arktis); Environment Canada; the Government of Nunavut, and the Vetlessen Foundation. The Nunavut Wildlife Management Board and the Polar Continental Shelf Program provided key logistical and financial supports for the KB collaring effort. This material is based on the project supported by the National Science Foundation Graduate Research Fellowship under Grant No. DGE-1256082; the University of Washington’s School of Aquatic and Fishery Sciences; and the US National Aeronautics and Space Administration’s (NASA) Biodiversity Program Grant No. NNX11A063G (PI: K. Laidre). Three anonymous reviewers helped to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Escajeda, E., Laidre, K.L., Born, E.W. et al. Identifying shifts in maternity den phenology and habitat characteristics of polar bears (Ursus maritimus) in Baffin Bay and Kane Basin. Polar Biol 41, 87–100 (2018). https://doi.org/10.1007/s00300-017-2172-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-017-2172-6