Abstract
Pleuragramma antarcticum is the dominant pelagic fish in the waters of the continental shelf in high Antarctic regions, where it plays a key role in the food web. A nursery ground for eggs of this species was first identified in 2002 in Terra Nova Bay (Ross Sea), where eggs were found trapped in ice platelets under the sea-ice during the spring. As part of a monitoring program aimed at understanding the geographic and temporal characteristics of this nursery ground, the present study reports on surveys carried out in the austral springs of 2005 and 2006 using a simple and effective method for sampling from the sea-ice. These surveys enabled the evaluation of the spatial range of the nursery area of the Antarctic silverfish in the sea-ice of the coastal area of Victoria Land between the Coulman Island and the Drygalski Glacier Tongue. P. antarcticum eggs were concentrated in an area of Terra Nova Bay of about 270 km2, encompassing two adjacent sites, Gerlache Inlet and Silverfish Bay. The present results add information on life cycle and hatching period of the Antarctic silverfish and confirm the importance of the Terra Nova Bay as a nursery area for this important species. Moreover, the survey points to the sea-ice cover and platelet ice as important environmental features of the nursery area.
Similar content being viewed by others
Introduction
The Antarctic silverfish Pleuragramma antarcticum plays a pivotal role in the high Antarctic coastal marine ecosystem, being one of the major links between lower and higher trophic levels (Eastman 2005; O’Driscoll et al. 2011). As the prevalent plankton feeder of the intermediate trophic level, this fish is considered a keystone species, much like Euphausia superba (the Antarctic krill) is for waters beyond the continental shelf (Guglielmo et al. 1998) and E. crystallorophias (the ice krill) is for the neritic zone (Vallet et al. 2011).
Among notothenioids, P. antarcticum is the only known holopelagic species, living all stages of its development within the water column, although, like other members of this suborder, it lacks a gas bladder. Evolution of the pelagic lifestyle involved a suite of adaptations including reduction in skeletal ossification and deposition of lipids (Eastman 1993, 1997; Klingenberg and Ekau 1996; Wöhrmann et al. 1997; Maes et al. 2006; Near et al. 2009; Albertson et al. 2010; La Mesa and Eastman 2011).
Adults are widely distributed in the coastal areas around the Antarctic continent, including the Scotia Arc and adjacent islands (Gerasimchuk 1986; DeWitt et al. 1990; Miller 1993; Knox 1994; Trunov 2001; Fuiman et al. 2002; Donnelly and Torres 2008). They inhabit both open waters and areas of pack ice and are generally found in mid-water at depths from 0 to 900 m (Gerasimchuk; 1986; DeWitt et al. 1990; Fuiman et al. 2002). In the Ross and Weddell Seas, this fish accounts for over 90 % of the local ichthyological community in terms of both number and biomass (Hubold and Ekau 1987; DeWitt 1970). With its wide distribution and high numbers, adults of P. antarcticum represent the primary food item for most Antarctic marine vertebrates including mammals, birds and other fishes (La Mesa et al. 2004).
Pleuragramma antarcticum reaches sexual maturity at 6 to 7+ years, at a length greater than about 130 mm SL (Faleyeva and Gerasimchuk 1990; Ferrando et al. 2010), and lays pelagic eggs, a characteristic known for only few species among notothenioids (Kellermann 1991; Kock and Kellermann 1991), with high fecundity values, between 4,315 and 17,774 eggs/female (Kock and Kellermann 1991). Spawning events have been predicted to occur at the end of austral winter, or at the beginning of spring, along the major continental ice shelves (Kellermann 1987; Hubold 1990; Faleyeva and Gerasimchuk 1990; Eastman 1993; Ferrando et al. 2010). Indirect evidence (Regan 1916; Kellermann 1989; Hubold 1990), and preliminary in situ observations (Vacchi et al. 2004), indicated hatching had occurred prior to November and December in colder coastal waters. However, Liu and Chen (1995) estimated a more prolonged incubation period, with spawning at the beginning of July and hatching 5–6 months later (in December), while DeWitt and Tyler (1960) estimated a longer breeding season for P. antarcticum in the Ross Sea, lasting from early October to late December. Eastman (1993) suggested that the spawning of P. antarcticum may be influenced by the hydrographic conditions related to stationary coastal polynyas.
Silverfish larvae and juveniles constitute the majority of ichthyoplankton at many locations around Antarctica (Hubold and Ekau 1987; Koubbi et al. 1997, 2011; Morales-Nin et al. 1998), including the western Ross Sea, where they may contribute to up to 98 % of the ichthyoplankton (Guglielmo et al. 1998; Vacchi et al. 1999; Granata et al. 2002).
In November 2002, embryonated eggs of P. antarcticum were detected floating in huge amounts among the subsurface platelet ice at Terra Nova Bay (TNB), Ross Sea, identifying for the first time a nursery and hatching area of the Antarctic silverfish (Vacchi et al. 2004). Since this time, a project aimed at monitoring the spawning area of the silverfish, in Terra Nova Bay, has been operating within the PNRA (Italian National Programme for Research in Antarctica).
An understanding of the geographic extent and the environmental characteristics of the nursery area are necessary to clarify the reproductive strategy of the silverfish and its life cycle. Such knowledge would also be useful to predict other possible reproductive sites along the Antarctic coast. Moreover, an understanding of whether the sea-ice cover in the nursery area is mostly an environmental constraint to be overcome, or whether it provides favorable conditions and contributes to the success of the silverfish early life history, is crucial. Appreciation of these points will help predict possible impacts of changes in the dynamics of the Antarctic sea-ice zone on this important species and has implications for the Antarctic ecosystem, where it plays a central role (see also Smith et al. 2007; Moline et al. 2008).
In the present work, we report the results of surveys carried out in the austral springs of 2005 and 2006 to check the spatial distribution and abundance of silverfish eggs in the sea-ice of coastal Victoria Land between Cape Washington (north) and the floating Drygalski Ice Tongue (south). A quick and effective sampling method through the ice cover was developed to enable extended geographical survey while minimizing environmental disturbance.
Materials and methods
The study area
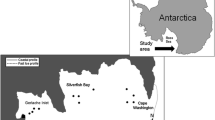
Terra Nova Bay is a coastal area of Victoria Land (western Ross Sea) near 75°S, 164°E, approximately 6,000 km2 in area (65 km north/south by 92 km east/west) (Fig. 1). It is delimited to the north by Cape Washington and to the south by the floating Drygalski Ice Tongue. The coastline is indented with numerous embayments including Gerlache Inlet and Silverfish Bay in its northern part. The Drygalski Ice Tongue, the Nansen Ice Sheet and the Campbell Glacier Tongue flow down from the continent into the Bay. The bottom topography is rather irregular and it is characterized by steep seabeds and by the Drygalski depression, a deep pit, elongated along shore, reaching more than 1,100 m (the greatest depth of the Ross Sea).
The marine environment of TNB is among the coldest in the world, due to its high latitude and the presence of large floating masses of continental ice. The seawater temperature is commonly at its freezing point (FP, −1.91 °C), with only rare excursions above −1.0 °C (Buffoni et al. 2002).
Summer circulation in TNB shows a prevailing northward direction in the upper layer along the coast with a clockwise rotation at depth. The coastal area is characterized by the presence of warmer and more saline water, while the lowest temperature values are found in the central area of the Bay, probably due both to local eddies and to upwelling processes determined by katabatic winds (Budillon and Spezie 2000; Buffoni et al. 2002).
A large polynya (mean area 1,300 km2, with a maximum open area up to 5,000 km2) is maintained during the winter by a combination of persistent katabatic winds, coupled with a barrier effect of the Drygalski Ice Tongue on pack ice advection coming from the south/southwest (Kurtz and Bromwich 1983, 1985).
The Terra Nova Bay polynya acts as an ice factory during winter and has a primary function in the sea-ice local dynamics (Van Woerst 1999). As a result, a major environmental feature of TNB is the seasonal sea-ice cover, bordering coastal areas for almost 9 months of the year. Platelet ice is present under the sea-ice cover, making up a semi-consolidated layer ranging from a few centimeters to meters in thickness. Detailed information on platelet ice distribution and seasonal dynamics in the area was not available at the time of our survey.
The Italian Mario Zucchelli Station (MZS) is located on a rocky promontory in the Gerlache Inlet area (Fig. 1) and it is open for the scientific research between late October and mid-February.
Sampling
The survey was carried out in springtime during the 2005 and 2006 Italian Antarctic expeditions. On the whole, the survey covered the seasonal period from the end of October to mid-December. Sampling was done by drilling holes through the sea-ice cover with a 15-cm-diameter hand-held auger. The use of hand auger was preferred to power motor augers since they were equally effective, but easier to transport. Moreover, the use of manual tools significantly reduced the environmental impact of the field activities.
Fish eggs and hatched larvae, floating among sea water and platelet ice after drilling, were collected by lowering a cylindrical PVC sampler into the ice hole. The sampler had a diameter of 10.5 cm and a total capacity of 7 L (Fig. 2). We considered the 7 L volume a representative subsample of the total volume (49.5 L, assuming a median depth of 2.8 m of consolidated ice) drained into the hole from the surrounding water, at the sea-ice under-face. The number of specimens counted in the 7 L subsample was normalized to 1 L and considered as a rough estimate of abundance in each station.
The field activity included a preliminary test in several sea-ice-covered stations, within a range of 230 km from MZS, reached either by snowmobile (stations placed within a radius of 15 km from MZS) or by helicopter. Following this preliminary study, the area between Cape Washington and Gerlache Inlet was chosen for intensive sampling. A grid of sampling stations placed 1–2 km from each other was monitored over the two seasons, with slight modifications of the sampling design due to interannual differences in extension and thickness of the sea-ice canopy. The geographic position of each station was determined in situ by GPS.
The length of the auger needed to reach the sea water through the ice was used to estimate the thickness of the consolidated sea-ice cap in each station. Bottom depth was measured by an echo sounder whose transducer was dipped in the hole, or it was inferred from the bathymetric lines of the nautical charts available for TNB and adjacent areas. The presence/absence of platelet ice was evaluated visually.
The samples were transferred into insulated boxes and returned to the laboratory at MZS. After filtering through a 500-μm mesh sieve to eliminate water, the samples were sorted: fish eggs, larvae and any other biological items were separated from the ice platelets and preserved in 4 % paraformaldehyde in seawater. Eggs and larvae were examined and counted under a stereomicroscope; identification was carried out according to Vacchi et al. (2004). As a rule, eggs and larvae of the whole 7-liter sample were counted. Some very abundant samples (>2,000 eggs/larvae) were further subsampled by a Folsom Plankton Splitter tool, according to Sell and Evans (1982).
The graphical representation of the abundance of P. antarcticum eggs and larvae was carried out with Surfer 9 software (Golden Software, Inc.)
During the 2005 Antarctic campaign, an additional sampling series was performed at the end of the main survey period, from the end of November to the middle of December. Sampling of eggs and larvae under the sea-ice with the PVC sampler was coupled with plankton net sampling. A collapsible plankton net (30 cm diameter, 200 μm mesh) was used to collect samples at two reference depth ranges (−70 to −20 m; −20 m to sea surface). Sampling was performed in one site in Silverfish Bay (74°35.877′S–164°37.667′E) from a sea-ice hole obtained by expanding a natural crack with a chainsaw and ice axe. In this site, a rough estimate of the abundance of eggs and larvae from every depth range was made. In addition, some basic biologic parameters (standard length, yolk sac presence and size) were evaluated to check the development of larvae.
Results
2005 survey
As a preliminary test, between October 27, 2005, and November 9, 2005, we monitored a large geographic area within 230 km of MZS, between the Coulman Island and the Drygalski Ice Tongue, by sampling at 41 stations. We found evidence of fish eggs under the ice in the northern part of TNB only, near the Campbell Glacier Tongue (Fig. 1; Table 1).
On the basis of this test, and considering the logistic constraints in working in the most remote geographic areas, we concentrated the following activities in the coastal region, between Gerlache Inlet and Cape Washington. From south to north, the following three locations were studied: GI (Gerlache Inlet), SB (Silverfish Bay), CW (W of Cape Washington) (Fig. 1). SB is a roughly triangular body of water in the middle of the investigated coastal region (between the Campbell Ice Tongue and the Oscar Point) named after the first finding of large amounts of silverfish eggs and larvae (SCAR Composite Gazetteer of Antarctica Id 18082).
SB and CW were covered by a thick consolidated sea-ice cap, and surveyed through ice holes from November 9, 2005, to December 01, 2005, along a grid of stations placed at 1–2 km from each other (Fig. 3). Conversely, as a consequence of strong katabatic winds that swept the area during the previous winter (http://www.pnra.meteo.it), GI waters were largely free from ice, and therefore the sampling was limited to sites where the sea-ice was sufficiently solid and safe.
Sampling stations with superimposed the abundances of silverfish eggs and larvae in the three geographic locations surveyed in 2005. White circles: sampling stations where eggs or larvae were absent; black circles: abundances of eggs and larvae; GI, Gerlache Inlet; SB, Silverfish Bay; CW, West of Cape Washington. The extent of sea-ice cover is indicated (gray area)
Fish eggs and larvae were found in 42 of the 90 stations sampled (Table 2). All eggs were P. antarcticum embryos at the final stage of development. The newly hatched larvae were identified as P. antarcticum according to size, morphology and pigmentation patterns described by Vacchi et al. (2004).
The abundance of fish eggs and larvae, the sea-ice thickness and the occurrence of platelet ice was different in the three locations (Table 2).
In the GI location, large amounts of embryonated silverfish eggs and larvae were only present near the western side of Campbell Glacier Tongue, where the sea-ice was thick and platelet ice was present.
By considering the percentage of positive samples, and the abundance values, Silverfish Bay emerged as an important location: fish eggs and newly hatched larvae were present under a canopy of thick consolidated sea-ice in a vast area, with a mean abundance of 74.98 (±91.74) eggs/larvae per liter. Platelet ice was present in most of the sampling stations in this locality.
At CW, the sea-ice cover was thinner and the occurrence of platelet ice was low and we found a low abundance of specimens in 2 of 14 sampling stations (Table 2).
A map of the distribution of abundances (Fig. 3) shows that in 2005, the nursery ground of P. antarcticum included a large part of the Silverfish Bay sea-ice, and a small portion of the Gerlache Inlet sea-ice, near the western side of Campbell Glacier Tongue with a surface area of at least 65 km2. In this nursery ground, the amounts of fish eggs/larvae peaked at 909 per liter, the sea-ice thickness was at least 2.4 m, and the sea depth over 300 m. Moreover, eggs and larvae occurred only where platelet ice was present beneath the under-surface of the sea-ice. In three samples, together with eggs and larvae, we found frozen fish remains, entrapped in the ice platelets; such remains were identified as parts of adult specimens of P. antarcticum by the distinctive hollow vertebral centra and scales (Vaillant 1906; Eastman and DeVries 1982; Von Busekist et al. 2007).
At the beginning of the survey, each sample was composed of a mixture of eggs and newly hatched larvae. The egg/larva ratio changed over time, however, with an increasing proportion of larvae from the second half of November. At the end of the survey, the larvae were more than 80 % of each sample (Fig. 4).
The additional sampling performed in 2005 from 27 November to 14 December showed no further eggs and only a few dead larvae to be present under the sea-ice in SB. Sampling with the plankton net revealed the presence of young silverfish larvae in the water column, with a very high abundance between −20 m and the sea surface under the ice. In fact, we collected 161 Pleuragramma larvae at this depth range, whereas only 44 individuals were obtained in deeper waters. The standard length of these larvae ranged between 7.5 and 12.5 mm, and the yolk sac was still present, although almost completely reabsorbed.
2006 survey
The 2006 survey (29 October–24 November) was designed to get a replicate of the sampling done during the previous year. Therefore, the same locations (GI, SB, CW) were studied, and the same sampling protocol and tools were used. However, more favorable conditions in sea-ice coverage and thickness, in particular within in Gerlache Inlet (GI), allowed coverage of a more extensive grid of stations (Fig. 5).
Sampling stations with superimposed the abundances of silverfish eggs and larvae in the three geographic locations surveyed in 2006. White circles: sampling stations where eggs or larvae were absent; black circles: abundances of eggs and larvae; GI, Gerlache Inlet; SB, Silverfish Bay; CW, West of Cape Washington. The extent of sea-ice cover is indicated (gray area)
Fish eggs and larvae were found in 71 of 93 sampled stations (Table 3); similar to the previous year, all of these samples were eggs at in the final stages of development mixed with newly hatched larvae of P. antarcticum.
In all the sampling locations, the abundance values of silverfish eggs/larvae were higher than those obtained in 2005.
In GI, which was extensively sampled during this second year of survey, fish eggs/larvae were found in 82.45 % of the stations with a mean abundance value of 133.87 (±215.66). Eggs and larvae were widespread in the area with the exception of its inner parts. The median value of sea-ice thickness was 2.4 m with ice platelets occurring at the majority of the stations (Table 3).
In SB, the percentage of spatial coverage was similar to what observed in 2005 (73.07 % of positive records), and the sea-ice thickness and occurrence of platelet ice were also similar to previous records; however, we registered here a very high concentration of silverfish eggs and larvae, with a mean value of 228.25 (±357.69) per liter (Table 3).
At CW, fish eggs and larvae were present in 50 % of samples, with a mean abundance of 7.90 (±17.33) (Table 3); the sea-ice thickness was similar to that measured in Silverfish Bay, but platelet ice was recorded in 3 stations only.
In the 2006 survey, fish eggs and larvae under the sea-ice were abundant and widely distributed in a vast area around the Campbell Glacier Tongue, including a large part of the sea-ice of Gerlache Inlet and Silverfish Bay (Fig. 5), covering an estimated area of almost 270 km2. The amounts of fish eggs/larvae were higher than in the 2005 survey, with values up to 1,100 per liter in Silverfish Bay. The sea-ice thickness was typically over 2.4 m, and the seabed depths were more than 200 m. As in the previous year, the presence of eggs and larvae correlated with the presence of platelet ice. Again, we found remains of adult P. antarcticum (mostly scales and frozen parts of the bodies in 10 samples).
Small percentages of newly hatched larvae were found at the beginning of the sampling period and they increased from the second half of November to reach a value of 60 % per sample at the end of November (Fig. 6).
Discussion
A number of studies carried out on the biology and ecology of the silverfish in various Antarctic sectors illustrate the important role that the silverfish plays in the Antarctic coastal ecosystem (e.g., Hubold 1984; Kellermann 1986; Donnelly et al. 2004; La Mesa et al. 2004; Granata et al. 2009; Koubbi et al. 2011; O’Driscoll et al. 2011; Vacchi et al. 2012). Terra Nova Bay is the first nursery area described for this important Antarctic fish species, with circum-Antarctic distribution. Significant number of silverfish embryonated eggs was first found at this locality in the spring of 2002, floating in huge amounts among the platelet ice layer under the sea-ice (Vacchi et al. 2004). This discovery has already led to significant progress in our understanding of the life cycle of the silverfish. For instance, it has allowed the confirmation of previous indirect evidence that P. antarcticum lays pelagic eggs (Marshall 1953; Faleyeva and Gerasimchuk 1990) and that reproduction occurs near-shore (Kellermann 1986). It also disproved the hypothesis of Kellermann (1996), which proposed demersal egg development, conversely pointing to adaptive needs that allow embryo development and larval survival in a challenging environment (Cziko et al. 2006; Bottaro et al. 2009; Evans et al. 2012). Due to the ecological relevance of the discovery, a long-term monitoring program was established within the PNRA in order to better understand both geographic and temporal characteristics of the nursery ground. In the present work, we provide a first assessment of the distribution and density of eggs and newly hatched larvae. The seasonal observations in two subsequent years (a) confirmed the importance of the Terra Nova Bay as a nursery area of the Antarctic silverfish, (b) pointed to the platelet ice as a relevant environmental feature of the nursery phase, and (c) added evidence about the hatching period and reproductive events.
Before discussing the above points, given the difficulty of sampling under the sea-ice, we believe that it could be useful to provide some comments on the methods used to collect eggs and larvae.
Sampling from the sea-ice
Sampling from ice-covered areas presents a number of difficulties not usually encountered in studies conducted in ice-free waters (Eicken et al. 2009). The presence of sea-ice coverage virtually precludes the possibility of performing horizontal net tows and also requires tools and time to cut holes through thick ice in order to gain access to the water. In addition, the possibility to sample organisms living in the sea-ice water interface is very difficult (Kirkwood and Burton 1987) especially when attempting quantitative estimates. Indeed, drilling a hole through the sea-ice cover induces disturbance at the ice/sea water interface. This, in turn, entails local hydrodynamic changes, resulting from the draining of sea water into the hole, which is proportional to the volume of the hole and to the compensating flow of water at the sea-ice underside (Dieckmann et al. 1992).
Methods and equipment used in previous studies to sample water and plankton from ice, such as “umbrella” and collapsible nets (Kirkwood and Burton 1987) or ADONIS (Dieckmann et al. 1992), appeared inadequate for our purposes, due to the presence of platelet ice. Diver-operated tools, like a suction sampler for sympagic fauna (Lønne 1988), were also not appropriate for our survey due to logistical constraints of diving in remote sea-ice areas. In comparison with other methodologies previously used (Kirkwood and Burton 1987; Magnuson and Stuntz 1970; Patterson et al. 1978; Dieckmann et al. 1992), the simple tool and procedures used by us allowed the survey to be undertaken rapidly over an extended area with minimum environmental disturbance. Thus, a large geographic area extending to 230 km from MZS, between the Coulman Island and the Drygalski Ice Tongue, was evaluated through 41 samples in less than 15 days (2005 survey), and a total of 224 samples were done in an overall period of about 2 months (1 month each year).
The importance of Terra Nova Bay as a nursery area of the Antarctic silverfish
Taken together, the results of our surveys indicate that silverfish embryonated eggs and newly hatched larvae were widely distributed under the sea-ice of an area of almost 270 km2 surrounding the Campbell Glacier Tongue, extending from the sea-ice of the Gerlache Inlet to Cape Washington. High quantities of eggs and larvae were present in two adjacent embayments of Terra Nova Bay encompassing both sides of the Campbell Ice Tongue, namely Gerlache Inlet (west of the Campbell Ice Tongue) and Silverfish Bay (on the eastern side of this glacier tongue) with abundance values of 96.84 (±200.16) per liter and 134.46 (±243.37) per liter, respectively. Silverfish Bay, the embayment encompassed by the Campbell Ice Tongue and Oscar Point, proved to be an important location within the nursery, with high spatial coverage and abundances up to 1,107 eggs/larvae per liter (mean rounded values 228.25 (±357.69) eggs/larvae per liter) registered in 2006. The area of CW is included in the nursery, but appeared less important in terms of abundance in both the 2 years.
It is worth noting that in all the studied stations, interannual differences in egg abundance were detected, with higher values in 2006. Moreover, in 2006, the eggs were not present in the in-shore parts of the nursery area. Such variation on an annual scale is probably related to variability of the physical and oceanographic characteristics in Terra Nova Bay and to the different extent of the sea-ice cover, which could influence egg dispersal from the spawning event on. In addition to interannual variability, the high standard deviation in abundances in each location indicates that the distribution of eggs is not homogenous, even over on small spatial scale. This is not surprising considering that eggs and newly hatched larvae are in a highly unstable environment where their local assemblage and distribution can be influenced by the morphology of underside of the sea-ice cover, by the organization of the platelet ice and by differences in local hydrodynamic conditions.
Platelet ice as a relevant feature of the nursery phase
Platelet ice consists of various-sized flat plate-like crystals up to about 10 cm in diameter, randomly oriented, mostly occurring under the coastal sea-ice cover (Gow et al. 1998; Leonard et al. 2006). Due to its prominent occurrence under sea-ice, platelet ice is considered a high latitude coastline feature, although its extent is still unknown. It can be incorporated into fast ice (Smith et al. 2001) or, more often, it aggregates loosely under fast or pack ice, making up a semi-consolidated layer that ranges from a few centimeters to several meters in thickness.
The mechanism of formation of platelet ice is far from being understood (McGuinness and Langhorne 2006; Crook 2010), but the influence of floating ice shelves or glacier tongues has been recognized to be important as they allow frazil ice to grow in the supercooled plumes formed nearby (Holland and Jenkins 1999).
Our results confirm that the occurrence of platelet ice at the sea-ice/sea water interface is a major feature of Terra Nova Bay (Van Woert 1999). Indeed the combination of the coastal oceanographic features, including the winter polynya, and the local topography shaped by the glacier tongues can explain both the wide distribution of the seasonal sea-ice cover and the occurrence of platelet ice beneath it. In addition to the oceanographic and ecological implications related to such a particular environment (Dieckmann et al. 1986; Arrigo and Thomas 2004; Thomas et al. 2010), platelet ice seems an important component of the nursery ground of the silverfish. Indeed, although we cannot exclude that eggs could be laid elsewhere, and passively accumulated in the platelet ice zone, eggs and larvae were always found mixed with ice platelets in the studied area. The occurrence of huge amounts of developing eggs in such a particular environment raises several questions. Is the sea-ice cover, and the associated platelet ice, an environmental constraint to be overcome by P. antarcticum, which spawns buoyant eggs near-shore in the winter? Conversely, does the platelet ice layer provide favorable ecological conditions to eggs, thus contributing to the success of the silverfish in its early life cycle? Moreover, since larvae of P. antarcticum have relatively low levels of antifreeze activity (Cziko et al. 2006; Evans et al. 2012), what are the adaptations that enable survival of embryos and emerging larvae in the challenging conditions associated with the platelet ice layer?
Sound answers to the above points are crucial in order to understand the degree of relationship of the silverfish with the coastal sea-ice, and the role of the silverfish in the cryopelagic community.
Although the relationship between the silverfish and the ice cover and platelet layer remains to be investigated fully, it is known that the silverfish life cycle is characterized by ontogenetic shifts in vertical/spatial distribution (Hubold and Ekau 1987; Granata et al. 2009; La Mesa et al. 2010; Koubbi et al. 2011; O’Driscoll et al. 2011) and that P. antarcticum is associated with sea-ice in various phases of its early life history (Granata et al. 2009; Kellermann 1986; Knox 1994; Vacchi et al. 2004, 2012). In particular, direct trophic interactions for early phases have been suggested on the basis of information on lipid seasonal reserves and foraging activity (Hubold and Hagen 1997; Koubbi et al. 2007), as well as on the basis of feeding of post-larvae on sea-ice-associated copepods (Granata et al. 2009).
The present data provide evidence that the silverfish life cycle begins very close to the surface sea-ice in spring and that eggs and newly hatched larvae become a locally dominant component of the cryopelagic community in an extended costal region of the Ross Sea (see Vacchi et al. 2012). The egg chorion acts as a protective barrier to embryos against the surrounding ice environment, while developmental adaptations in newly hatched larvae render them less likely to be at risk from freezing (Cziko et al. 2006; Bottaro et al. 2009). Experimental results by Evans et al. (2012) suggest that the high potential risk of exposure of emerging larvae to the icy conditions of the platelet layer could be minimized by a combination of behavioral adaptations such as developmental descent of eggs (sinking prior to hatch) and active swimming away from the surface ice. Our observations in the field support the sinking of newly hatched larvae in the water column. Indeed, the results of sampling with the plankton net performed in late November to beginning of December attest the presence of a large amount of young larvae in the water column.
On the other hand, the underside of sea-ice and the platelet ice layer are sites of enhanced primary production and provide favorable conditions for hatching and protection for several invertebrate grazing species whose life cycle is coupled with sea-ice formation and melting (Arrigo et al. 2010; Bluhm et al. 2010; Caron and Gast 2010). It has been suggested that the structure of irregularly disk-shaped ice platelets may also provide a favorable environment for the early life stages of the silverfish protecting them from predation (La Mesa et al. 2010). Moreover, a significant responsiveness in embryos sampled in the platelet layers toward the increase in pro-oxidant conditions naturally occurring in platelet ice could reflect the capability to safely live in this environment (Regoli et al. 2005).
Hatching time and spawning of the silverfish
A mixture of eggs at the latest stage of development and newly hatched larvae were present in each sample from the beginning of our survey. Even assuming that a small percentage of eggs hatch in each of the samples as the result of mechanical/thermal shock during sampling and transferring to the laboratory, a trend is recognizable of increasing hatching during the season, with the number of hatched larvae notably higher in the second half of November. Hatching seems to be concluded at the beginning of December, when no more eggs or newly hatched larvae are present under the sea-ice cover. According to these results, the hatching period is predominantly in November, with massive hatching events in the second half of that month. Our results agree with previous studies, which hypothesize hatching of the silverfish in colder coastal waters prior to December (Regan 1916; Kellermann 1989; Hubold 1990; Vacchi et al. 2004). However, present data are still not sufficient to clarify the development period for silverfish eggs and to back-date the spawning time. Nonetheless, previous hypotheses (Hubold 1984; Faleyeva and Gerasimchuk 1990; Vacchi et al. 2004) and some recent evidence based on the histological gonadic condition of a Ross Sea silverfish population (Ferrando et al. 2010) are consistent with spawning occurring in mid winter (July–August), thus giving a development time for the eggs of four-five months.
Since spawning events have not been directly observed in the field, the spawning behavior of the silverfish remains to be elucidated. Migration to coastal areas along the major continental ice shelves for reproduction has been predicted on various lines of evidence (e.g., Kellermann 1987; Faleyeva and Gerasimchuk 1990; Hubold 1990; Eastman 1993; La Mesa et al. 2010; Moline et al. 2008).
The occurrence of huge numbers of embryonated eggs all at similar stages of development, together with evidence of a massive hatching, described in the present work, is suggestive of a mass spawning event in these northern parts of the coastal region of the Ross Sea. The finding of remains of adult silverfish specimens trapped in the ice in both years of survey gives further support to this possible scenario, implying that the locality may serve as both a spawning and a nursery area. Whether the eggs are released and fertilized very close to the sea-ice cover (remaining in a challenging environment for all the embryonic development period) or are laid in deeper ice-free seawater rising through their natural buoyancy and possible local hydrodynamic flows to reach the icy nursery environment after fertilization (Vacchi et al. 2012) are alternative hypotheses to be tested by further research.
An intriguing behavioral hypothesis has been recently proposed by Koubbi et al. (2011) who have suggested “homing” as a possible strategy helping adult silverfish to recognize the spawning sites during their winter migration to coastal areas. Accordingly, to this hypothesis, the adults would return to spawn at the same place where they were born, driven by a combination of geographical and environmental conditions experienced early in their life. Due to interannual environmental variations, a homing strategy may not lead to eggs being released in optimal areas every year, but it might ensure a good larval survival rate over the long term. However, the occurrence of such a homing mechanism may create a time lag in the detectable impact of long-term environmental change on spawning distribution, thus stressing the importance of performing long-term monitoring surveys to predict the possible effects of environmental changes (see also Vacchi et al. 2012 and references therein). Although homing behavior remains possible, progressing our knowledge of the characteristics that make Terra Nova Bay a suitable area for spawning and nursery of the silverfish remains a priority objective to understand the ecology and life cycle of this important Antarctic species and to make sound predictions about possible impacts of environmental changes on the coastal Antarctic ecosystem (see also Smith et al. 2007; Moline et al. 2008; Vacchi et al. 2012).
References
Albertson C, Yi-Lin Yan YL, Titus TA, Pisano E, Vacchi M, Yelick PC, Detrich HW, Postlethwait JH (2010) Molecular pedomorphism underlies craniofacial skeletal evolution in Antarctic notothenioid fishes. BMC Evol Biol 10:4
Arrigo KR, Thomas DN (2004) Large scale importance of sea ice biology in the Southern Ocean. Antarct Sci 16:471–486
Arrigo KR, Mock T, Lizotte MP (2010) Primary producers and sea ice. In: Thomas DN, Dieckmann GS (eds) Sea ice, 2nd edn. Blackwell, Oxford, pp 283–325
Bluhm BA, Gradinger RR, Schnack-Schiel SB (2010) Sea ice meio and macrofauna. In: Thomas DN, Dieckmann GS (eds) Sea ice, 2nd edn. Blackwell, Oxford, pp 357–393
Bottaro M, Oliveri D, Ghigliotti L, Pisano E, Ferrando S, Vacchi M (2009) Born among the ice: first morphological observations on two developmental stages of the Antarctic silverfish Pleuragramma antarcticum, a key species of the Southern Ocean. Rev Fish Biol Fish 19:249–259
Budillon G, Spezie G (2000) Thermohaline structure and variability in Terra Nova Bay polynya, Ross Sea. Antarct Sci 12:493–508
Buffoni G, Cappelletti A, Picco P (2002) An investigation of thermohaline circulation in Terra Nova Bay polynya. Antarct Sci 14:83–92
Caron DA, Gast RJ (2010) Heterotrophic protists associated with sea ice. In: Thomas DN, Dieckmann GS (eds) Sea ice, 2nd edn. Blackwell, Oxford, pp 327–356
Crook J (2010) Ice growth and platelet crystals in Antarctica. PhD Thesis, Victoria University of Wellington, New Zealand
Cziko PA, Evans CW, Cheng C-HC, DeVries AL (2006) Freezing resistance of antifreeze-deficient larval Antarctic fish. J Exp Biol 209:407–420
DeWitt HH (1970) The character of the midwater fish fauna of the Ross Sea, Antarctica. In: Holdgate MW (ed) Antarctic ecology, vol 1. Academic Press, London, pp 305–314
DeWitt HH, Tyler JC (1960) Fishes of the Stanford Antarctic Biological Research Program, 1959–1959. Stanford Ichthyol Bull 7:162–199
DeWitt HH, Heemstra PC, Gon O (1990) Nototheniidae. In: Gon O, Heemstra PC (eds) Fishes of the Southern Ocean. JLB Smith Institute of Ichthyology, Grahamstown, pp 279–331
Dieckmann GS, Rohardt G, Hellmer H, Kipfstuhl J (1986) The occurrence of ice platelets at 250 m near the Filchner Ice Shelf and its significance to sea ice biology. Deep-Sea Res 33:141–148
Dieckmann GS, Arrigo K, Sullivan CW (1992) A high-resolution sampler for nutrient and chlorophyll a profiles of the sea ice platelet layer and underlying water column below fast ice in polar oceans. Preliminary results. Mar Ecol Prog Ser 80:291–300
Donnelly J, Torres JJ (2008) Pelagic fishes in the Marguerite Bay region of the West Antarctic Peninsula shelf. Deep Sea Res II 55:523–539
Donnelly J, Torres JJ, Sutton TT, Simoniello C (2004) Fishes of the eastern Ross Sea, Antarctica. Polar Biol 27:637–650
Eastman JT (1993) Antarctic fish biology: evolution in an unique environment. Academic Press, San Diego
Eastman JT (1997) Phyletic divergence and specialization for pelagic life in the Antarctic Notothenioid fish Pleuragramma antarcticum. Comp Biochem Physiol A 118:1095–1101
Eastman JT (2005) The nature of the diversity of Antarctic fishes. Polar Biol 28:93–107
Eastman JT, DeVries AL (1982) Buoyancy studies of notothenioid fishes in McMurdo Sound, Antarctica. Copeia 2:385–393
Eicken H, Gradinger R, Salganek M, Shirasawa K, Perovich D, Leppäranta M (2009) Field techniques for Sea ice research. University of Alaska Press, Fairbanks
Evans CW, Williams DE, Vacchi M, Brimble MA, DeVries AL (2012) Metabolic and behavioural adaptations during early development of the Antarctic silverfish. Pleuragramma antarcticum. Polar Biol. doi:10.1007/s00300-011-1134-7
Faleyeva TI, Gerasimchuk VV (1990) Features of reproduction in the Antarctic sidestripe, Pleuragramma antarcticum (Nototheniidae). J Ichthyol 30:67–79
Ferrando S, Hanchet S, Angiolillo M, Gambardella C, Pisano E, Tagliaferro G, Vacchi M (2010) Insights into the life cycle of the Antarctic Silverfish. Reproduction features of the Ross Sea population, International Polar Year Oslo Science Conference, June 2010, abstracts, p 1
Fuiman LA, Davis RW, Williams TM (2002) Behavior of midwater fishes under Antarctic ice: observations by a predator. Mar Biol 140:815–822
Gerasimchuck VV (1986) Characteristics of Antarctic silverfish, Pleuragramma antarctica (Nototheniidae), from Olaf-Pruds Bay (Commonwealth Sea, Eastern Antarctica) with notes on the identification of the species. J Ichthyol 26:10–17
Gow AJ, Ackley SF, Govoni JW (1998) Physical and structural properties of land-fast ice in McMurdo Sound, Antarctica. In: Jeffries MO (ed) Antarctic Sea ice: physical processes, interactions and variability. Antarct Res Ser 74 American Geophysical Union, Washington DC, pp 355–374
Granata A, Cubeta A, Gugliemo L, Sidoti O, Greco S, Vacchi M, La Mesa M (2002) Ichthyoplankton abundance and distribution in the Ross Sea during 1987–1996. Polar Biol 25:87–202
Granata A, Zagami G, Vacchi M, Guglielmo L (2009) Summer and spring trophic niche of larval and juvenile Pleuragramma antarcticum in the western Ross Sea, Antarctica. Polar Biol 32:369–382
Guglielmo L, Granata A, Greco S (1998) Distribution and abundance of postlarval and juvenile Pleuragramma antarcticum (Pisces, Nototheniidae) off Terra Nova Bay (Ross Sea, Antarctica). Polar Biol 19:37–51
Holland DM, Jenkins JA (1999) Modelling thermodynamic ice-ocean interactions at the base of an ice shelf. J Phys Oceanogr 29:1787–1800
Hubold G (1984) Spatial distribution of Pleuragramma antarcticum (Pisces: Nototheniidae) near the Filchner and Larsen ice shelves (Weddell Sea, Antarctica). Polar Biol 3:231–236
Hubold G (1990) Seasonal patterns of ichthyplankton distribution and abundance in the Southern Weddell Sea. In: Kerry KR, Hempel G (eds) Antarctic ecosystems: ecological change and conservation. Springer, Berlin, pp 149–158
Hubold G, Ekau W (1987) Midwater fish fauna of the Weddell Sea, Antarctica. In: Kullander SO, Fernholm B (eds) Proceedings of the fifth congress of European ichthyologists. Swedish Museum of Natural History, Stockholm, pp 391–396
Hubold G, Hagen W (1997) Seasonality of feeding and lipid content in juvenile Pleuragramma antarcticum (Pisces: Nototheniidae) from the southern Weddell Sea. In: Battaglia B, Valencia J, Walton WH (eds) Antarctic communities. Species, structure and survival. Cambridge University Press, New York, pp 277–283
Kellermann A (1986) On the biology of early life stages of notothenioid fishes (Pisces) off the Antarctic Peninsula. Ber Polarforsch 31:1–155
Kellermann A (1987) Food and feeding ecology of postlarval and juvenile Pleuragramma antarcticum (Pisces; Notothenioidei) in the seasonal pack ice zone off the Antarctic Peninsula. Polar Biol 7:307–315
Kellermann A (1989) The larval fish community in the zone of seasonal ice cover and its seasonal and interannual variability. Arch Fischereiweiss 39:89–109
Kellermann A (1991) Eggs and larval drift of the Antarctic fish Notothenia coriiceps. Cybium 15:199–210
Kellermann A (1996) Midwater fish ecology. In: Ross RM, Hofmann EE, Quetin LB (eds.) Foundations for ecological research West of the Antarctic Peninsula. Antarct Res Ser 70. American Geophysical Union, Washington, DC, pp 231–256
Kirkwood JM, Burton HR (1987) Three new zooplankton nets designed for under-ice sampling; with preliminary results of collections made from Ellis Fjord, Antarctica during 1985. Proc NIPR Symp Polar Biol 1:112–122
Klingenberg CP, Ekau W (1996) A combined morphometric and phylogenetic analysis of an ecomorphological trend: pelagization in Antarctic fishes (Perciformes: Nototheniidae). Biol J Linn Soc 59:143–177
Knox GA (1994) The biology of the Southern Ocean. Cambridge University Press, Cambridge
Kock KH, Kellermann A (1991) Reproduction in Antarctic notothenioid fish. Antarct Sci 3:125–150
Koubbi P, Hureau JC, Vacchi M, White M (1997) Results of the preliminary survey on the coastal distribution of fish larvae in Adelie Land (Southern Ocean) during January–February 1996. Cybium 21:381–392
Koubbi P, Vallet C, Razouls S, Grioche A, Hilde D, Courcot L, Janquin MA, Vacchi M, Hureau JC (2007) Condition and diet of larval Pleuragramma antarcticum from Terre Adélie (Antarctica) during summer. Cybium 31:67–76
Koubbi P, O’Brien C, Loots C, Giraldo C, Smith M, Tavernier E, Vacchi M, Vallet C, Chevallier J, Moteki M (2011) Spatial distribution and inter-annual variations in the size frequency distribution and abundances of Pleuragramma antarcticum larvae in the Dumont d’Urville Sea from 2004 to 2010. Polar Sci 5:225–238
Kurtz DD, Bromwich DH (1983) Satellite observed behaviour of the Terra Nova Bay polynya. J Geophys Res 88:9717–9722
Kurtz DD, Bromwich DH (1985) A recurring, atmospherically forced polynya in Terra Nova Bay. In: Jacobs SS (ed.) Oceanology of the Antarctic continental shelf. Antarct Res Ser 43, American Geophysical Union, Washington DC, pp 177–201
La Mesa M, Eastman JT (2011) Antarctic silverfish: life strategies of a key species in the high-Antarctic ecosystem. Fish Fish. doi:10.1111/j.1467-2979.2011.00427.x
La Mesa M, Eastman JT, Vacchi M (2004) The role of notothenioid fish in the food web of the Ross Sea shelf waters: a review. Polar Biol 27:321–338
La Mesa M, Catalano B, Russo A, Greco S, Vacchi M, Azzali M (2010) Influence of environmental conditions on spatial distribution and abundance of early life stages of Antarctic silverfish, Pleuragramma antarcticum (Nototheniidae), in the Ross Sea. Antarct Sci 22:243–254
Leonard GH, Purdie CR, Langhorne PJ, Haskell TG, Williams MJM, Frew RD (2006) Observations of platelet ice growth and oceanographic conditions during the winter of 2003 in McMurdo Sound. Antarctica, J Geophys Res 111
Liu Q, Chen DG (1995) Length frequency analysis of Pleuragramma antarcticum, Electrona antarctica, Protomyctophum bolini. Chin J Oceanol Limnol 13:380–384
Lønne OJ (1988) A diver-operated electric suction sampler for sympagic (= under-ice) invertebrates. Pol Res 6:135–136
Maes J, Van de Putte A, Hecq JH, Volckaert FAM (2006) State dependent energy allocation in the pelagic Antarctic silverfish Pleuragramma antarcticum: trade-off between winter reserves and buoyancy. Mar Ecol Prog Ser 326:269–282
Magnuson JJ, Stuntz WE (1970) A siphon water sampler for use through the ice. Limnol Oceanogr 15:156–158
Marshall NB (1953) Egg size in Arctic, Antarctic and Deep-Sea fishes. Evolution 7:328–341
McGuinness MJ, Langhorne P (2006) A platelet puzzle in Antarctica. In: Proceedings of KSIAM 2006 annual meeting, Nov 24–25, Konkuk University, Seoul
Miller RG (1993) A history and atlas of the fishes of the Antarctic Ocean. Foresta Institute for Ocean and Mountain Studies, Carson City
Moline MA, Karnovsky NJ, Brown Z, Divoky GJ, Frazer TK, Jacoby CA, Torres JJ, Fraser WR (2008) High latitude changes in ice dynamics and their impact on polar marine ecosystems. Ann NY Acad Sci 1134:267–319
Morales-Nin B, Garcia MA, Lopez O (1998) Distribution of larval and juvenile Nototheniops larseni and Pleuragramma antarcticum off the Antarctic Peninsula in relation to oceanographic conditions. Cybium 22:69–81
Near TJ, Jones CJ, Eastman JT (2009) Geographic intraspecific variation in buoyancy within Antarctic notothenioid fishes. Antarct Sci 21:123–129
O’Driscoll RL, Macaulay GJ, Gauthier S, Pinkerton M, Hanchet S (2011) Distribution, abundance and acoustic properties of Antarctic silverfish (Pleuragramma antarcticum) in the Ross Sea. Deep Sea Res II 58:181–195
Patterson RJ, Dykes L, Frape S, McLeod RA, Wickens J (1978) A new method for collecting water samples from beneath the ice. Limnol Oceanogr 23:1029
Regan CT (1916) Larval and postlarval fishes. British Antarctic (“Terra Nova”) Expedition 1910. Nat Hist Rep Zool 1:125–156
Regoli F, Nigro M, Benedetti M, Fattorini D, Gorbi F (2005) Antioxidant efficiency in early life stages of the Antarctic silverfish, Pleuragramma antarcticum: responsiveness to pro-oxidant conditions of platelet ice and chemical exposure. Aquat Toxicol 75:43–52
Sell DW, Evans MS (1982) A statistical analysis of subsampling and an evaluation of the Folsom plankton splitter. Hydrobiologia 94:223–230
Smith IJ, Langhorne PJ, Haskell TJ, Trodahl HJ, Frew R, Venner R (2001) Platelet ice and the land-fast sea ice of McMurdo Sound, Antarctica. Ann Glaciol 33:21–27
Smith WO, Ainley DG, Cattaneo-Vietti R (2007) Marine ecosystems: the Ross Sea. Antarctic ecology: from genes to ecosystems. Phil Trans R Soc B 362:95–111
Thomas DN, Papadimitriou S, Michel C (2010) Biogeochemistry of Sea Ice. In: Thomas DN, Dieckmann GS (eds) Sea ice, 2nd edn. Blackwell, Oxford, pp 425–467
Trunov IA (2001) Occurrence of Pleuragramma antarcticum (Nototheniidae) off South Georgia Island and the South Sandwich Islands (Antarctica). J Ichthyol 41:549–550
Vacchi M, La Mesa M, Greco S (1999) Summer distribution and abundance of larval and juvenile fishes in the western Ross Sea. Antarct Sci 11:54–60
Vacchi M, La Mesa M, Dalù M, Macdonald M (2004) Early life stages in the life cycle of Antarctic silverfish, Pleuragramma antarcticum in Terra Nova Bay, Ross Sea. Antarct Sci 16:299–305
Vacchi M, Koubbi P, Ghigliotti L, Pisano E (2012) Sea-Ice interactions with polar fish—focus on the Antarctic Silverfish Life History. In: Verde C, di Prisco G (eds.) Adaptation and evolution in marine environments, vol 1, From Pole to Pole. Springer, Berlin, pp 51–73
Vaillant ML (1906) Poissons. Expedition Antarctique Francaise 1903–1905. Masson et Cie, Editeurs, Paris
Vallet C, Labat JP, Smith M, Koubbi P (2011) Interannual variations in Euphausiid life stage distribution in the Dumont d’Urville Sea from 2004 to 2008. Polar Sci 5:166–178
Van Woert ML (1999) Wintertime dynamics of the Terra Nova Bay polynya. J Geophys Res 104:1169–7753
Von Busekist J, Vacchi M, Albertelli G (2007) “ANFIBO Base”, a computer-based system for identification of fish bones from Antarctic waters. Version 1.0 for MS-Windows CD-ROM. http://www.mna.it, University of Genoa, Italy
Wöhrmann APA, Hagen W, Kunzmann A (1997) Adaptations of the Antarctic silverfish Pleuragramma antarcticum (Pisces: Nototheniidae) to pelagic life in High Antarctic waters. Mar Ecol Prog Ser 151:205–218
Acknowledgments
This work was carried out within the project ECOFISH, supported by Italian National Programme for Research in Antarctica (PNRA project 2004/8.04). We thank John MacDonald for his input and Mike Taler and Federico Mazzei for their help and companionship in the field. Sara Ferrando helped in the preparation of figures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vacchi, M., DeVries, A.L., Evans, C.W. et al. A nursery area for the Antarctic silverfish Pleuragramma antarcticum at Terra Nova Bay (Ross Sea): first estimate of distribution and abundance of eggs and larvae under the seasonal sea-ice. Polar Biol 35, 1573–1585 (2012). https://doi.org/10.1007/s00300-012-1199-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-012-1199-y