Abstract
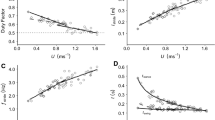
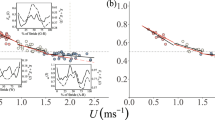
Juvenile animals often suffer from high levels of predation. Development of an effective and efficient locomotor system is therefore likely to be crucial towards ensuring their survival. However, our understanding of locomotor efficiency, at least in terms of energetic cost in young animals is poor. We performed this study as Svalbard rock ptarmigan, Lagopus muta hyperborea must rapidly develop the ability to locomote prior to the onset of their first winter, during which conditions are extreme. To aid survival, adult ptarmigan deposit large winter fat stores, whilst at the same time males exhibit a reduced metabolic cost of locomotion. Sub-adult males, however, are unable to fully acquire fat stores during their first winter and the maturity of their locomotor systems is unknown. Here, we investigate the energetics and kinematics of terrestrial locomotion in sub-adult male birds using flow-through respirometry and high-speed video recordings, respectively. We demonstrate that in terms of running speed and metabolic cost, sub-adult ptarmigan develop a mature functioning locomotor system prior to the onset of winter. This research indicates that achieving a mature locomotor system allows young males to emerge from the winter with the ability to compete for territories and mates during the breeding season.


Similar content being viewed by others
References
Blix AS (2005) Arctic animals and their adaptations to life on the edge. Tapir Academic Press, Trondheim
Breitwisch R, Diaz M, Lee R (1987) Foraging efficiencies and techniques of juvenile and adult northern mockingbirds (Mimus polyglottos). Behaviour 101:225–235
Brody S (1945) Bioenergetics and growth. Reinhold, New York
Carrier DR (1996) Ontogenetic limits on locomotor performance. Physiol Zool 69:467–488
Dial KP, Jackson BE (2011) When hatchlings outperform adults: locomotor development in Australian brush turkeys (Alectura lathami, Galliformes). Proc R Soc B 278:1610–1616
Fedak MA, Rome L, Seeherman HJ (1981) One-step N2-dilution technique for calibrating open-circuit V02 measuring systems. Am Physiol Soc 51:772–776
Goldstein DL (1988) Estimates of daily energy-expenditure in birds—the time-energy budget as an integrator of laboratory and field studies. Am Zool 28:829–844
Grammeltvedt R, Steen JB (1978) Fat deposition in Spitzbergen ptarmigan (Lagopus mutus hyperboreus). Arctic 31:496–498
Herrel A, Gibb AC (2006) Ontogeny of performance in vertebrates. Physiol Biochem Zool 79:1–6
Jackson BE, Segre P, Dial KP (2009) Precocial development of locomotor performance in a ground-dwelling bird (Alectoris chukar): negotiating a three-dimensional terrestrial environment. Proc R Soc B 276:3457–3466
Kram R, Taylor CR (1990) Energetics of running: a new perspective. Nature 346:265–267
Lack D (1954) The natural regulation of animal numbers. Oxford University Press, Oxford
Lees JJ, Nudds RL, Stokkan K-A, Folkow LP, Codd JR (2010) Reduced metabolic cost of locomotion in Svalbard rock ptarmigan (Lagopus muta hyperborea) during winter. PLoS ONE 5(11):e15490
Lighton JRB (2008) Measuring metabolic rates: a manual for scientists. Oxford University Press, New York
Marchetti K (1989) Differences in the foraging of juvenile and adult birds: the importance of developmental constraints. Biol Rev 64:51
Metcalfe NB, Monaghan P (2001) Compensation for a bad start: grow now, pay later? Trends Ecol Evol 16:254–260
Mortensen A, Unander S, Kolstad M, Blix AS (1983) Seasonal changes in body composition and crop content of Spitzbergen ptarmigan Lagopus mutus hyperboreus. Ornis Scand 14:144–148
Nudds RL, Folkow LP, Lees JJ, Tickle PG, Stokkan K-A, Codd JR (2011) Evidence for energy savings from aerial running in the Svalbard rock ptarmigan (Lagopus muta hyperborea). Proc R Soc B 278:2654–2661
Parker H, Ottesen H, Knudsen E (1985) Age determination in Svalbard ptarmigan Lagopus mutus hyperboreus. Polar Res 3:125–126
Pedersen HC, Steen JB, Andersen R (1983) Social organization and territorial behaviour in a Willow ptarmigan population. Ornis Scand 14:263–272
Pedersen AO, Overrein O, Unander S, Fuglei E (2005) Svalbard Rock Ptarmigan (Lagopus mutus hyperboreus) a status report. Norsk Polarinstitutt 125:1–20
Prestrud P, Nilssen K (1992) Fat deposition and seasonal variation in body composition of Arctic foxes in Svalbard. J Wildl Manage 56:221–233
Prestrud P, Nilssen K (1995) Growth, size, and sexual dimorphism in Arctic foxes. J Mammal 76:522–530
Recher HF, Recher JA (1969) Comparative foraging efficiency of adult and immature little blue herons (Florida caerulea). Anim Behav 17:320–322
Reierth E, Stokkan K-A (1998) Activity rhythm in high Arctic Svalbard ptarmigan (Lagopus mutus hyperboreus). Can J Zool 76:2031–2039
Reimers E (1982) Winter mortality and population trends of reindeer on Svalbard, Norway. Arct Alp Res 14:295–300
Reimers E, Ringberg T (1983) Seasonal changes in body weights of Svalbard reindeer from birth to maturity. Acta Zool Fenn 175:69–72
Ricklefs RE (1973) Patterns of growth in birds. 2. Growth-rate and mode of development. Ibis 115:177–201
Ricklefs RE (1979a) Adaptation, constraint, and compromise in avian postnatal development. Biol Rev Camb Philos Soc 54:269–290
Ricklefs RE (1979b) Patterns of growth in birds. 5. A comparative study of development in the starling, common tern, and Japanese quail. Auk 96:10–30
Ricklefs RE, Shea RE, Choi IH (1994) Inverse relationship between functional maturity and exponential growth rate of avian skeletal muscle: a constraint on evolutionary response. Evolution 48:1080–1088
Rubenson J, Heliams DB, Lloyd DG, Fournier PA (2004) Gait selection in the ostrich: mechanical and metabolic characteristics of walking and running with and without an aerial phase. Proc R Soc B 271:1091–1099
Sahlman T, Segelbacher G, Hoglund J (2009) Islands in the ice: colonisation routes for rock ptarmigan to the Svalbard archipelago. Ecography 32:840–848
Steen JB, Unander S (1985) Breeding biology of the Svalbard rock ptarmigan Lagopus mutus hyperboreus. Ornis Scand 16:191–197
Stokkan K-A, Sharp PJ, Unander S (1986) The annual breeding cycle of the high-Arctic Svalbard ptarmigan (Lagopus mutus hyperboreus). Gen Comp Endocrinol 61:446–451
Stokkan K-A, Sharp PJ, Dunn IC, Lea RW (1988) Endocrine changes in photostimulated willow ptarmigan (Lagopus lagopus lagopus) and Svalbard ptarmigan (Lagopus mutus hyperboreus). Gen Comp Endocrinol 70:169–177
Stokkan K-A, Lindgard K, Reierth E (1995) Photoperiodic and ambient temperature control of the annual body mass cycle in Svalbard ptarmigan. J Comp Physiol [B] 165:359–365
Tickle PG, Richardson MF, Codd JR (2010) Load carrying during locomotion in the barnacle goose (Branta leucopsis): the effect of load placement and size. Comp Biochem Physiol A Mol Integr Physiol 156(3):309–317
Tolkamp BJ, Emmans GC, Yearsley J, Kyriazakis I (2002) Optimization of short-term animal behaviour and the currency of time. Anim Behav 64:945–953
Unander S, Steen JB (1985) Behavior and social-structure in Svalbard rock ptarmigan Lagopus mutus hyperboreus. Ornis Scand 16:198–204
Wassersug RJ, Sperry DG (1977) Relationship of locomotion to differential predation on Pseudacris triseriata (Anura: Hylidae). Ecology 58:830–839
Watson A (1965) A population study of Ptarmigan (Lagopus mutus) in Scotland. J Anim Ecol 34:135–172
Acknowledgments
We would like to thank Magnus Folkow, Hans Lian, Hans-Arne Solvang and John Ness for technical assistance and animal husbandry during these experiments. We would also like to acknowledge Robert Nudds for statistical advice. This research was funded by the Biotechnology and Biological Science Research Council (BBSRC) (G01138/1) grant to J. Codd. J. Lees was supported by a Natural Environment Research Council (NERC) PhD doctoral training award.
Conflict of interest
No conflicts of interest, financial or otherwise are declared by the authors.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lees, J.J., Stokkan, KA., Folkow, L.P. et al. Locomotor development in the Svalbard rock ptarmigan (Lagopus muta hyperborea). Polar Biol 35, 867–874 (2012). https://doi.org/10.1007/s00300-011-1131-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-011-1131-x