Abstract
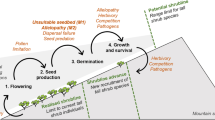
Populations of the two native vascular plant species on the Antarctic Peninsula have increased over the past 40 years. This increase has been attributed to improved reproductive performance resulting from regional warming and increased growing season length. However, little is known of the influence that vascular plants have on the performance of neighboring plants in developing and well-established communities. We compared the aboveground growth and reproduction of Deschampsia antarctica plants growing alone or in close proximity to neighboring plants (D. antarctica, Colobanthus quitensis, or mosses) at a young, recently colonized and an older, well-developed plant community on the Antarctic Peninsula to assess whether neighboring plants had a positive or negative effect on D. antarctica performance, and whether these effects varied from young to old communities. In both communities, tillers on D. antarctica plants near neighbors produced 48–89% fewer leaves and 49–93% fewer tillers than those on D. antarctica plants growing alone. These tillers also had relative growth rates that were 25–66% lower- and tiller-size indices that were 42–87% less than those on plants growing alone. In addition, the biomass of tillers on plants growing near neighbors was 40–91% lower than those on plants growing alone. Leaf and tiller production was generally higher in the older, more developed community than in the younger community. Our findings illustrate that vegetative growth of D. antarctica is reduced when growing in close proximity to neighboring plants, suggesting that negative plant interactions are an important constraint at our field sites.




Similar content being viewed by others
References
Aguiar MR, Soriano A, Sala OE (1992) Competition and facilitation in the recruitment of seedlings in Patagonian steppe. Funct Ecol 6:66–70
Arroyo MTK, Cavieres LA, Peñaloza A, Arroyo-Kalin MA (2003) Positive associations between the cushion plant Azorella monantha (Apiaceae) and alpine plant species in the Chilean Patagonian Andes. Plant Ecol 169:121–129
Bradshaw AW (1993) Understanding the fundamentals of succession. In: Miles J, Walton DH (eds) Primary succession on land. Blackwell, Oxford, pp 1–4
Brancaleoni L, Strelin J, Gerdol R (2003) Relationships between geomorphology and vegetation patterns in subantarctic and Andean tundra of Tierra del Fuego. Polar Biol 26:404–410
Callaway RM (1995) Positive interactions among plants. Bot Rev 61:306–349
Callaway RM (1997) Positive interactions in plant communities and the individualistic-continuum concept. Oecologia 12:143–149
Callaway RM, Walker LR (1997) Competition and facilitation: a synthetic approach to interaction in plant communities. Ecology 78:1958–1965
Callaway RM, Brooker RW, Choler P, Kikvidez Z, Lortie CJ, Michalet R, Paolini L, Pugnaire FI, Newingham B, Aschehoug ET, Armas C, Kikodze D, Cook BJ (2002) Positive interactions among alpine plants increase with stress. Nature 417:844–848
Carlsson BA, Callaghan TV (1991) Positive plant interactions in tundra vegetation and the importance of shelter. J Ecol 79:973–983
Cavieres LA, Arroyo MTK, Peñaloza A, Molina-Montenegro M, Torres C (2002) Nurse effect of Bolax gummifera cushion plants in the alpine vegetation of the Chilean Patagonian Andes. J Veg Sci 13:547–554
Cavieres LA, Badano EI, Sierra-Almeida A, Gómez-González S, Molina-Montenegro MA (2006) Positive interactions between alpine plant species and the nurse cushion plant Laretia acaulis do not increase with elevation in the Andes of central Chile. New Phytol 69:59–69
Cavieres LA, Badano EI, Sierra-Almeida A, Gómez-González S, Molina-Montenegro MA (2007) Microclimatic modifications of cushion plants and their consequences for seedling survival of native and non-native herbaceous species in the high Andes of central Chile. Arct Alp Res 39:229–236
Chapin FS, Walker LR, Fastie CL, Sharman LC (1994) Mechanisms of primary succession following deglaciation at Glacier Bay, Alaska. Ecol Mon 64:149–175
Choler P, Michalet R, Callaway RM (2001) Facilitation and competition on gradients in alpine plant communities. Ecology 82:3295–3308
Convey P, Smith RIL (2006) Responses of terrestrial Antarctic ecosystems to climate change. Plant Ecol 182:1–10
Cook AJ, Fox AJ, Vaughan DG, Ferrigno JG (2005) Retreating glacier fronts on the Antarctic Peninsula over the past half-century. Science 308:541–544
Day TA, Ruhland CT, Grobe CW, Xiong F (1999) Growth and reproduction of Antarctic vascular plants in response to warming and UV radiation reductions in the field. Oecologia 119:24–35
Day TA, Ruhland CT, Xiong FS (2008) Warming increases aboveground biomass and C stocks in vascular-plant-dominated Antarctic tundra. Global Change Biol 14:1827–1843
Egerton JJG, Wilson SD (1993) Plant competition over winter in alpine shrubland and grassland, Snowy Mountains, Australia. Arct Alp Res 25:124–129
Fowbert JA, Smith RIL (1994) Rapid population increases in native vascular plants in the Argentine Islands, Antarctic Peninsula. Arct Alp Res 26:290–296
Gerdol R, Brancaleoni L, Marchesini R, Bragazza L (2002) Nutrient and carbon relations in subalpine dwarf shrubs after neighbor removal or fertilization in northern Italy. Oecologia 130:476–483
Gerighausen UTA, Brautigam K, Mustafa O, Peter HU (2003) Expansion of vascular plants on an Antarctic island—a consequence of climate change? In: Huiskes AHL, Gieskes WWC, Rozema J, Schorno RML, van der Vies SM, Wolf WJ (eds) Antarctic biology in a global context. Backhuys, Netherlands, pp 79–83
Grobe CW, Ruhland CT, Day TA (1997) A new population of Colobanthus quitensis near Arthur Harbor, Antarctica: Correlating recruitment with warmer summer temperatures. Arct Alp Res 29:217–221
Hobbie SE, Shevtsova A, Chapin S (1999) Plant responses to species removal and experimental warming in Alaskan tussock tundra. Oikos 84:417–434
Jones GA, Henry GHR (2003) Primary plant succession on recently deglaciated terrain in the Canadian High Arctic. J Biogeogr 30:277–296
McGraw JB, Garbutt K (1990) Demographic growth analysis. Ecology 71:1199–1204
Moen J (1993) Positive versus negative interactions in a high alpine block field: germination of Oxyria digyna seeds in a Ranunculus glacialis community. Arct Alp Res 25:201–206
Nuñez CI, Aizen MA, Ezcurra C (1999) Species associations and nurse plant effects in patches of high-Andean vegetation. J Veg Sci 10:357–364
Olofsson J, Moen J, Oksanen L (1999) On the balance between positive and negative plant interactions in harsh environments. Oikos 86:539–543
Park JH, Day TA, Strauss S, Ruhland CT (2007) Biogeochemical pools and fluxes of carbon and nitrogen in a maritime tundra near penguin colonies along the Antarctic Peninsula. Polar Biol 30:199–207
Parnikoza I, Convey P, Dykyy I, Trokhymets V, Milinevsky G, Tyschenko O, Inozemtesva D, Kozeretska I (2009) Current status of the Antarctic herb tundra formation in the Central Argentine Islands. Glob Change Biol. doi:10.11/j1365-2486.2009.01906
Ruhland CT, Xiong FS, Clark WD, Day TA (2005) The influence of ultraviolet-B radiation on growth, hydroxycinnamic acids and flavonoids of Deschampsia antarctica during springtime ozone depletion in Antarctica. Photochem Photobiol 81:1086–1093
Smith RIL (1994) Vascular plants as bioindicators of regional warming in Antarctica. Oecologia 99:322–328
Smith RIL (1996) Terrestrial and freshwater biotic components of the western Antarctic Peninsula. In: Ross RM, Hoffman EE, Quetin LB (eds) Foundations for ecological research west of the Antarctic Peninsula (Antarctic Research Series, vol 70). American Geophysical Union, Washington, pp 15–59
Smith RIL, Poncet S (1987) Deschampsia antarctica and Colobanthus quitensis in the Terra Firma Islands. Br Antarct Surv Bull 74:31–35
Smith RC, Ainley D, Baker K, Domack E, Emslie S, Fraser B, Kennett J, Laventer A, Mosely-Thompson E, Stammerjohn S, Vernet M (1999) Marine ecosystem sensitivity to climate change. Bioscience 49:393–404
Sonesson M, Callaghan TV (1991) Strategies of survival in plants of Fennoscandian tundra. Arctic 44:95–105
Statistix (1996) Statistics for windows. User’s Manual. Analytical Software, Tallahassee
Theodose TA, Bowman WD (1997) The influence of interspecific competition on the distribution of an alpine graminoid: Evidence for the importance of plant competition in an extreme environment. Oikos 79:101–114
Turner J, Colwell SR, Marshall GJ, Lachlan-Cope TA, Carleton AM, Jones PD, Lagun V, Reid PA, Iagovkina S (2005) Antarctic climate change during the last 50 years. Int J Climatol 25:204–279
Vaughn DG, Marchall GJ, Connolley WM, King JC, Mulvaney R (2001) Devil in the detail. Science 293:1777–1779
Vaughn DG, Marchall GJ, Connolley WM, Pakinson C, Mulvaney R, Hodgson DA, King JC, Pudsey CJ, Turner J (2003) Recent rapid regional climate warming on the Antarctic Peninsula. Clim Change 60:243–274
Walker LR, Chapin FS (1987) Interactions among processes controlling succession. Oikos 50:131–135
Walker LR, Zasada JC, Chapin FS (1986) The role of life history processes in primary succession on an Alaskan floodplain. Ecology 67:1243–1253
Wilson SD (1993) Competition and resource availability in heath and grassland in the Snowy Mountains of Australia. J Ecol 81:445–451
Wilson SD (1999) Plant interactions during secondary succession. In: Walker LR (ed) Ecosystems of disturbed ground (ecosystems of the world). Elsevier, Amsterdam, pp 611–632
Xiong FS, Ruhland CT, Day TA (1999) Photosynthetic temperature response of the Antarctic vascular plants Colobanthus quitensis and Deschampsia antarctica. Physiol Plant 106:276–286
Xiong FS, Mueller EC, Day TA (2000) Photosynthetic and respiratory acclimation and growth response of Antarctic vascular plants to contrasting temperature regimes. Am J Bot 87:700–710
Acknowledgments
This research was supported by the National Science Foundation Office of Polar Programs (grant # OPP-0230579) and a Summer Research Grant to CTR from The College of Graduate Studies and Research at MNSU. We especially thank Caroline De Van for her assistance in the field. We also thank the science and support personnel at Palmer Station for their help. Logistical support was provided by Raytheon Polar Services Company.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krna, M.A., Day, T.A. & Ruhland, C.T. Effects of neighboring plants on the growth and reproduction of Deschampsia antarctica in Antarctic tundra. Polar Biol 32, 1487–1494 (2009). https://doi.org/10.1007/s00300-009-0646-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-009-0646-x