Abstract
In spite of their dominance in meltwater environments in the Polar Regions, little is known about conditions that control community structure and production in microbial mats. Microbial mats were sampled at 13 recently separated ponds on the McMurdo Ice Shelf, Antarctica, with the aim to determine microbial mat community response to shifts in deterministic processes. Community structure in ponds of different size classes responded to different environmental variables, with conductivity as a common theme. Biomass and net oxygen exchange did not vary across the pond conditions and may reflect the very slow turnover rates characteristic of Antarctic microbial communities. Microbial mat communities on the MIS appear unresponsive to large intra-annual variability, while the long-term inter-annual physiochemical environment of the overlying appears to be influencing the community dynamics.





Similar content being viewed by others
References
APHA (1998) Standard methods for the analysis of water and wastewater, 20th edn. American Public Health Association, Washington
Barber HG, Haworth EY (1981) A guide to the morphology of the diatom frustule. Freshwater Biological Association
Battarbee RW, Charles DF, Dixit SS, Renberg I (1999) Diatoms as indicators of surface water acidity. In: Stoermer EF, Smol J (eds) The diatoms: applications for the environmental and earth sciences. Cambridge University Press, London, pp 85–127
Bebout B (1992) Interactions of nitrogen and carbon cycling in microbial mats and stromatolites. Ph.D. dissertation. Curriculum in Marine Sciences, University of North Carolina, Chapel Hill
Broady P, Kibblewhite A (1991) Morphological characterization of Oscillatoria (Cyanobacteria) from Ross Island and southern Victoria Land, Antarctica. Antarct Sci 3:35–45. doi:10.1017/S095410209100007X
Campbell IB, Claridge GGC (1987) Antarctica: soils, weathering processes and environment. Developments in soil science, vol 16. Elsevier, New York
Clarke KR, Ainsworth M (1993) A method of linking multivariate community structure to environmental variables. Mar Ecol Prog Ser 92:205–219. doi:10.3354/meps092205
Clarke KR, Warwick RM (2001) Changes in marine communities. An approach to statistical analysis and interpretation, 2nd edn. Plymouth Marine Laboratory, UK
Cremer H, Gore D, Hultzsch N, Melles M, Wagner B (2004) The diatom flora and limnology of lakes in the Amery Oasis, East Antarctica. Polar Biol 27:513–531. doi:10.1007/s00300-004-0624-2
de los Ríos A, Ascaso C, Wierzchos J, Fernández-Valiente E (2004) Microstructural characterization of cyanobacterial mats from the McMurdo Ice Shelf, Antarctica. Appl Environ Microbiol 70:569–580. doi:10.1128/AEM.70.1.569-580.2004
De Mora SJ, Whitehead RF, Gregory M (1991) The chemical composition of glacial melt water ponds and steams on the McMurdo Ice Shelf, Antarctica. Antarct Sci 6:17–27
Downes M (2001) NIWA Christchurch auto-analyser methods. NIWA internal report no. 103. NIWA, Christchurch
Fallu M, Allaire N, Pienitz R (2000) Freshwater diatoms from northern Quebec and Labrador (Canada). Species-environment relationships in lakes of boreal forest, forest-tundra and tundra regions. Bibl Diatomol 45:1–200
Fritz SC, Cumming BF, Gasse F, Laird KR (1999) Diatoms as indicators of hydrologic and climatic change in saline lakes. In: Stoermer EF, Smol J (eds) The diatoms: applications for the environmental and earth sciences. Cambridge University Press, London, pp 41–72
Hawes I, Howard-Williams C, Vincent WF (1992) Desiccation and recovery of Antarctic cyanobacterial mats. Polar Biol 12:587–594. doi:10.1007/BF00236981
Hawes I, Howard-Williams C, Pridmore RD (1993) Environmental control of microbial biomass in the ponds of the McMurdo Ice Shelf, Antarctica. Arch Hydrobiol 127:271–287
Hitzfeld B, Lampert C, Späth N, Mountfort D, Kaspar H, Dietrich D (2000) Toxin production in cyanobacterial mats from ponds on the McMurdo Ice Shelf, Antarctica. Toxicon 38:1731–1748. doi:10.1016/S0041-0101(00)00103-3
Howard-Williams C, Hawes I (2007) Ecological processes in Antarctic inland waters: interactions between physical processes and the nitrogen cycle. Antarct Sci 19:205–217. doi:10.1017/S0954102007000284
Howard-Williams C, Pridmore R, Downes MT, Vincent WF (1989) Microbial biomass, photosynthesis and chlorophyll a related pigments in the ponds of the McMurdo Ice Shelf, Antarctica. Antarct Sci 1:125–131. doi:10.1017/S0954102089000192
Howard-Williams C, Pridmore RD, Broady PA, Vincent WF (1990) Environmental and biological variability in the McMurdo Ice Shelf ecosystem. In: Kerry KR, Hempel G (eds) Antarctic ecosystems: ecological change and conservation. Springer, Berlin, pp 23–31
Jungblut A-D, Hawes I, Mountfort D, Hitzfeld B, Dietrich DR, Burns BP, Neilan BA (2005) Diversity within cyanobacterial mat communities in variable salinity meltwater ponds of McMurdo Ice Shelf, Antarctica. Environ Microbiol 7:519–529. doi:10.1111/j.1462-2920.2005.00717.x
Kellogg TB, Kellogg DE (2002) Non-marine and littoral diatoms from Antarctic and subantarctic regions––distribution and updated taxonomy. In: A. Witkowski (ed) Diatom monographs. ARG. Gantner Verlag KG
Komàrek J (2007) Phenotype diversity of the cyanobacteria genus Leptolyngbya in maritime Antarctica. Polar Res 28:211–231
Komàrek J, Anagnostidis K (2005) Cyanoprokaryota 2. Teil/2nd part: Oscillatoriales. In: Büdel B, Krienitz L, Gärtner G, Schagerl M (eds) Süsswasserflora von Mitteleuropa, Band 19/2. Elsevier, Munchen, pp 1–759
Komàrek J, Elster J, Komàrek O (2008) Diversity of the cyanobacteria microflora of the northern part of James Ross Island, NW Weddell Sea, Antarctica. Polar Biol 31:853–865. doi:10.1007/s00300-008-0424-1
Krammer K, Lange-Bertalot H (1991) Bacillariophyceae. 4. Teil: Achanathaceae, Kritische Erganzungen zu Navicula (Lineolatae) und Gomphonema. In: Ettl H, Gäntner G, Gerloff J, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa, Band 2/4. Gustav Fisher Verlag, Stuttgart, pp 1–437
Krammer K, Lange-Bertalot H (1997a) Bacillariophyceae. 1. Teil: Naviculaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa, Band 2/1. Spektrum Akademischer Verlag, Heidelberg, pp 1–876
Krammer K, Lange-Bertalot H (1997b) Bacillariophyceae. 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa, Band 2/1. Spektrum Akademischer Verlag, Heidelberg, pp 1–611
Krammer K, Lange-Bertalot H (2000) Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In: Ettl H, Gärtner G, Gerloff J, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa, Band 2/3. Gustav Fisher Verlag, Stuttgart, pp 1–599
Laing TE, Smol JP (2000) Factors influencing diatom distributions in circumpolar treeline lake of northern Russia. J Phycol 36:1035–1048. doi:10.1046/j.1529-8817.2000.99229.x
Marker AFH, Crowther CA, Gunn RJM (1980) Methanol and acetone as solvents for estimating chlorophyll a and phaeopigments by spectrophotometery. Arch Hydrobiol Ergeb Limnol 14:52–69
Mueller DR, Vincent WF, Bonilla S, Laurion I (2005) Extremotrophs, extremeophiles and broadband pigmentation strategies in a high arctic ice shelf ecosystem. FEMS Microbiol Ecol 53:73–87. doi:10.1016/j.femsec.2004.11.001
Nadeau TL, Milbrandt EC, Castenholz RW (2001) Evolutionary relationships of cultivated Antarctic Oscillatorians (Cyanobacteria). J Phycol 37:650–654. doi:10.1046/j.1529-8817.2001.037004650.x
Pinckney J, Paerl HW, Bebout BM (1995) Salinity control of benthic microbial mat community production in a Bahamian hypersaline lagoon. J Exp Mar Biol Ecol 187:223–237. doi:10.1016/0022-0981(94)00185-G
Quesada A, Fernández-Valiente E, Hawes I, Howard-Williams C (2008) Benthic primary production in polar lakes and rivers. In: Vincent WF, Laybourn-Parry J (eds) Polar lakes and rivers. Oxford University Press, USA, pp 179–196
Sabbe K, Verleyen E, Hodgson D, Vanhoutte K, Vyverman W (2003) Benthic diatom flora of freshwater and saline lakes in the Larsemann Hills and Rauer Islands, East Antarctica. Antarct Sci 15:227–248. doi:10.1017/S095410200300124X
Schmidt SW, Moskal W, De Mora S-J, Howard-Williams C, Vincent WF (1991) Limnological properties of Antarctic ponds during winter freezing. Antarct Sci 3:379–388. doi:10.1017/S0954102091000482
Spaulding SA, McKnight DM, Stoerner EF, Doran PT (1997) Diatoms in sediments of perennially ice-covered Lake Hoare, and implications for interpreting lake history in the McMurdo Dry Valleys of Antarctica. J Paleolimnol 17:404–420
Spaulding SA, Kociolek JP, Wong D (1999) A taxonomic and systematic revision of the genus Muelleria (Bacillariophyta). Phycologia 38:314–341
Spaulding S, Esposito R, Lubinski D, Horn S, Cox M, McKnight D, Alger A, Hall B, Mayernick M, Whittaker T, Yang C (2008) Antarctic freshwater diatoms web site, McMurdo Dry Valleys LTER. http://huey.colorado.edu/diatoms/
Taton A, Grubisic S, Balthasart P, Hodgson DA, Laybourn-Parry J, Wilmotte A (2006) Biogeographical distribution and ecological ranges of benthic cyanobacteria in East Antarctic lakes. FEMS Microbiol Ecol 57:272–289. doi:10.1111/j.1574-6941.2006.00110.x
Vincent WF (2000) Cyanobacterial dominance in the polar regions. In: Whitton BA, Potts M (eds) Ecology of the cyanobacteria: their diversity in space and time. Kluwer, Dordrecht, pp 321–340
Vincent WF, Mueller DR, Bonilla S (2004) Ecosystems on ice: the microbial ecology of Markham Ice Shelf in the high Arctic. Cryobiology 48:103–112. doi:10.1016/j.cryobiol.2004.01.006
Wait BR, Webster-Brown JG, Brown KL, Healy M, Hawes I (2006) PChemistry and stratification of Antarctic meltwater ponds 1: coastal ponds near Bratina Island, McMurdo Ice Shelf. Antarct Sci 18:515–524. doi:10.1017/S0954102006000563
Webster JG, Brown KL, Vincent WF (1994) Geochemical processes affecting meltwater chemistry and the formation of saline ponds in the Victoria Valley and Bull Pass region, Antarctica. Hydrobiologia 281:171–186
Acknowledgments
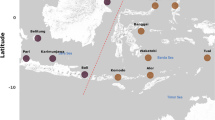
This study was funded and supported by the New Zealand Foundation for Research, Science and Technology (CO1605) and Antarctica New Zealand. Ian Hawes is thanked for his assistance in the field. Map and pond diagram was prepared by Greg Kelly. Dr. Clive Howard-Williams and three anonymous reviews provided useful comments that helped strengthen this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sutherland, D.L. Microbial mat communities in response to recent changes in the physiochemical environment of the meltwater ponds on the McMurdo Ice Shelf, Antarctica. Polar Biol 32, 1023–1032 (2009). https://doi.org/10.1007/s00300-009-0601-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-009-0601-x