Abstract
Key message
MANNANASE7 gene in Brassica napus L. encodes a hemicellulose which located at cell wall or extracellular space and dehiscence-resistance can be manipulated by altering the expression of MANNANASE7.
Abstract
Silique dehiscence is an important physiological process in plant reproductive development, but causes heavy yield loss in crops. The lack of dehiscence-resistant germplasm limits the application of mechanized harvesting and greatly restricts the rapeseed (Brassica napus L.) production. Hemicellulases, together with cellulases and pectinases, play important roles in fruit development and maturation. The hemicellulase gene MANNANASE7 (MAN7) was previously shown to be involved in the development and dehiscence of Arabidopsis (Arabidopsis thaliana) siliques. Here, we cloned BnaA07g12590D (BnMAN7A07), an AtMAN7 homolog from rapeseed, and demonstrate its function in the dehiscence of rapeseed siliques. We found that BnMAN7A07 was expressed in both vegetative and reproductive organs and significantly highly expressed in leaves, flowers and siliques where the abscission or dehiscence process occurs. Subcellular localization experiment showed that BnMAN7A07 was localized in the cell wall. The biological activity of the BnMAN7A07 protein isolated and purified through prokaryotic expression system was verified to catalyse the decomposition of xylan into xylose. Phenotypic studies of RNA interference (RNAi) lines revealed that down-regulation of BnMAN7A07 in rapeseed could significantly enhance silique dehiscence-resistance. In addition, the expression of upstream silique development regulators is altered in BnMAN7A07-RNAi plants, suggesting that a possible feedback regulation mechanism exists in the regulation network of silique dehiscence. Our results demonstrate that dehiscence-resistance can be manipulated by altering the expression of hemicellulase gene BnMAN7A07, which could provide an available genetic resource for breeding practice in rapeseed which is beneficial to mechanized harvest.





Similar content being viewed by others
References
Alonso-Cantabrana H, Ripoll JJ, Ochando I, Vera A, Ferrándiz C, Martínez-Laborda A (2007) Common regulatory networks in leaf and fruit patterning revealed by mutations in the Arabidopsis ASYMMETRIC LEAVES1 gene. Development (Cambridge, England) 134:2663–2671. https://doi.org/10.1242/dev.02864
Arnaud N, Girin T, Sorefan K, Fuentes S, Wood TA, Lawrenson T, Sablowski R, Ostergaard L (2010) Gibberellins control fruit patterning in Arabidopsis thaliana. Genes Dev 24(19):2127–2132. https://doi.org/10.1101/gad.593410
Arnim A (2007) Subcellular localization of GUS- and GFP-tagged proteins in onion epidermal cells. Cold Spring Harb Protoc. https://doi.org/10.1101/pdb.prot4689
Ballester P, Ferrandiz C (2017) Shattering fruits: variations on a dehiscent theme. Curr Opin Plant Biol 35:68–75. https://doi.org/10.1016/j.pbi.2016.11.008
Bemer M, van Mourik H, Muino JM, Ferrandiz C, Kaufmann K, Angenent GC (2017) FRUITFULL controls SAUR10 expression and regulates Arabidopsis growth and architecture. J EXP BOT 68(13):3391–3403. https://doi.org/10.1093/jxb/erx184
Bowman JL, Baum SF, Eshed Y, Putterill J, Alvarez J (1999) Molecular genetics of gynoecium development in Arabidopsis. Curr Top Dev Biol 45:155–205. https://doi.org/10.1016/s0070-2153(08)60316-6
Braatz J, Harloff HJ, Emrani N, Elisha C, Heepe L, Gorb SN, Jung C (2018) The effect of INDEHISCENT point mutations on silique shatter resistance in oilseed rape (Brassica napus). Theor Appl Genet 131(4):959–971. https://doi.org/10.1007/s00122-018-3051-4
Carrillo-Barral N, Matilla AJ, Rodríguez-Gacio MC, Iglesias-Fernández R (2018) Mannans and endo-β-mannanase transcripts are located in different seed compartments during Brassicaceae germination. Planta 247:649–661. https://doi.org/10.1007/s00425-017-2815-4
Chauvaux N, Child R, John K, Ulvskov P, Borkhardt B, Prinsen E, Van Onckelen HA (1997) The role of auxin in cell separation in the dehiscence zone of oilseed rape pods. J EXP BOT 48(7):1423–1429. https://doi.org/10.1093/jxb/48.7.1423
Child RN, John K, Ulvskov P, Van OH, Van Onckelen HA (1998) Ethylene biosynthesis in oilseed rape pods in relation to pod shatter. J EXP BOT 49(322):829–838. https://doi.org/10.1093/jxb/49.322.829
Child RD, Summers JE, Babij J, Farrent JW, Bruce DM (2003) Increased resistance to pod shatter is associated with changes in the vascular structure in pods of a resynthesized Brassica napus line. J EXP BOT 54(389):1919–1930. https://doi.org/10.1093/jxb/erg209
Degan FD, Child R, Svendsen I, Ulvskov P (2001) The cleavable N-terminal domain of plant endopolygalacturonases from clade B may be involved in a regulated secretion mechanism. J Biol Chem 276(38):35297–35304. https://doi.org/10.1074/jbc.M102136200
Dinneny JR, Weigel D, Yanofsky MF (2005) A genetic framework for fruit patterning in Arabidopsis thaliana. Development 132(21):4687–4696. https://doi.org/10.1242/dev.02062
Dong Y, Wang YZ (2015) Seed shattering: from models to crops. Front Plant Sci 6:476. https://doi.org/10.3389/fpls.2015.00476
Ferrandiz C (2002) Regulation of fruit dehiscence in Arabidopsis. J EXP BOT 53(377):2031–2038. https://doi.org/10.1093/jxb/erf082
Ferrandiz C, Pelaz S, Yanofsky MF (1999) Control of carpel and fruit development in Arabidopsis. Annu Rev Biochem 68:321–354. https://doi.org/10.1146/annurev.biochem.68.1.321
Ferrándiz C, Liljegren SJ, Yanofsky MF (2000) Negative regulation of the SHATTERPROOF genes by FRUITFULL during Arabidopsis fruit development. Science 289(5478):436–438. https://doi.org/10.1126/science.289.5478.436
Flanagan CA, Hu Y, Ma H (2010) Specific expression of the AGL1 MADS-box gene suggests regulatory functions in Arabidopsis gynoecium and ovule development. Plant J 10(2):343–353. https://doi.org/10.1046/j.1365-313X.1996.10020343.x
Girin T, Sorefan K, Ostergaard L (2009) Meristematic sculpting in fruit development. J EXP BOT 60(5):1493–1502. https://doi.org/10.1093/jxb/erp031
Gu Q, Ferrándiz C, Yanofsky MF, Martienssen R (1998) The FRUITFULL MADS-box gene mediates cell differentiation during Arabidopsis fruit development. Development 125(8):1509–1517. https://doi.org/10.1007/s004290050143
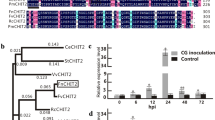
He HJ, Bai M, Tong PP, Hu YT, Yang M, Wu H (2018) CELLULASE6 and MANNANASE7 affect cell differentiation and Silique Dehiscence. Plant Physiol 176(3):2186–2201. https://doi.org/10.1104/pp.17.01494
Iglesias-Fernández R, Rodríguez-Gacio MC, Barrero-Sicilia C, Zalduegui PC, Matilla AJ (2011) Three endo-β-mannanase genes expressed in the micropylar endosperm and in the radicle infuence germination of Arabidopsis thaliana seeds. Planta 233:25–36. https://doi.org/10.1007/s00425-010-1257-z
Jaradat MR, Ruegger M, Bowling A, Butler H, Cutler AJ (2014) A comprehensive transcriptome analysis of silique development and dehiscence in Arabidopsis and Brassica integrating genotypic, interspecies and developmental comparisons. GM Crops Food 5(4):302–320. https://doi.org/10.4161/21645698.2014.947827
Jenkins ES, Paul W, Craze M, Whitelaw CA, Weigand A, Roberts JA (2010) Dehiscence-related expression of an Arabidopsis thaliana gene encoding a polygalacturonase in transgenic plants of Brassica napus. Plant Cell Environ 22(2):159–167. https://doi.org/10.1046/j.1365-3040.1999.00372.x
Kuai J, Sun YY, Liu TT, Zhang PP, Zhou M, Wu JS, Zhou GS (2016) Physiological mechanisms behind differences in Pod Shattering Resistance in Rapeseed (Brassica napus L.) varieties. PLoS ONE 11(6):e0157341. https://doi.org/10.1371/journal.pone.0157341
Kadkol GP, Macmillan RH, Burrow RP, Halloran GM (1984) Evaluation of Brassica genotypes for resistance to shatter. I. Development of a laboratory test. Euphytica 33(1):63–73. https://doi.org/10.1007/BF00022751
Karimi M, Inze D, Depicker A (2002) GATEWAYTM vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7(5):193–195. https://doi.org/10.1016/S1360-1385(02)02251-3
Koncz C, Schell J (1986) The promoter of TL-DNA gene 5 controls the tissue-specific expression of Chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet 204:383–396. https://doi.org/10.1007/BF00331014
Laga B, Den BB, Lambert B (2012) Brassica Plant Comprising a Mutant Indehiscent Allele. U.S. Patent No 9475849. Washington, DC: U.S. Patent and Trademark Office
Laga B, Den BB, Lambert B (2016) Brassica Plant Comprising a Mutant Indehiscent Allele. U.S. Patent No 8809635. Washington, DC: U.S. Patent and Trademark Office
Lambert B, Denolf P, Engelen S, Golds T, Haesendonckxa B, Ruiter R, Robbens S, Bots M, Laga B (2015) Omics-directed reverse genetics enables the creation of new productivity traits for the vegetable oil crop canola. Proc Environ Sci 29:77–78. https://doi.org/10.1016/j.proenv.2015.07.167
Li YM, Zhu JQ, Xu LZ, Zhao Z (2012) Experiment on strength of rapeseed pod dehiscence based on impending fracturing method. Nongye Gongcheng Xuebao/Trans Chinese Soc Agric Eng 28(8):111–115. https://doi.org/10.3969/j.issn.1002-6819.2012.08.017
Liljegren SJ, Ditta GS, Eshed Y, Savidge B, Bowman JL, Yanofsky MF (2000) SHATTERPROOF MADS-box genes control seed dispersal in Arabidopsis. Nature 404(6779):766–770. https://doi.org/10.1038/35008089
Liljegren SJ, Roeder AHK, Kempin SA, Gremski K, Ostergaard L, Guimil S, Reyes DK, Yanofsky MF (2004) Control of fruit patterning in Arabidopsis by INDEHISCENT. Cell 116(6):843–853. https://doi.org/10.1016/S0092-8674(04)00217-X
Liu J, Zhou RJ, Wang WX, Wang H, Qiu Y, Raman R, Mei DS, Raman H, Hu Q (2020) A copia like-retrotransposon insertion in the upstream region of SHATTERPROOF 1 gene, BnSHP1.A9 is associated with quantitative variation in pod shattering resistance in oilseed rape. J EXP BOT. https://doi.org/10.1093/jxb/eraa281
Ma H, Yanofsky MF, Meyerowitz EM (1991) AGL1-AGL6, an Arabidopsis gene family with similarity to floral homeotic and transcription factor genes. Genes Dev 5(3):484–495. https://doi.org/10.1101/gad.5.3.484
Marsch-Martinez N, Zuniga-Mayo VM, Herrera-Ubaldo H, Ouwerkerk PB, Pablo-Villa J, Lozano-Sotomayor P, Greco R, Ballester P, Balanza V, Kuijt SJ, Meijer AH, Pereira A, Ferrandiz C, de Folter S (2014) The NTT transcription factor promotes replum development in Arabidopsis fruits. Plant J 80(1):69–81. https://doi.org/10.1111/tpj.12617
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Biochem 31(3):426–426. https://doi.org/10.1021/ac60147a030
Mitsuda N, Ohme-Takagi M (2008) NAC transcription factors NST1 and NST3 regulate pod shattering in a partially redundant manner by promoting secondary wall formation after the establishment of tissue identity. Plant J Cell Mol Biol 56:768–778. https://doi.org/10.1111/j.1365-313X.2008.03633.x
Modrusan Z, Reiser L, Feldmann KA, Fischer RL, Haughn GW (1994) Homeotic transformation of ovules into carpel-like structures in Arabidopsis. Plant Cell 6(3):333. https://doi.org/10.1105/tpc.6.3.333
Morgan CL, Bruce DM, Child R, Ladbrooke ZL, Arthur AE (1998) Genetic variation for pod shatter resistance among lines of oilseed rape developed from synthetic B. napus. Field Crops Res. https://doi.org/10.1016/s0378-4290(98)00099-9
Ogawa M, Kay P, Wilson S, Swain SM (2009) ARABIDOPSIS DEHISCENCE ZONE POLYGALACTURONASE1 (ADPG1), ADPG2, and QUARTET2 are Polygalacturonases required for cell separation during reproductive development in Arabidopsis. Plant Cell 21(1):216–233. https://doi.org/10.1105/tpc.108.063768
Peng PF, Li YC, Mei DS, Liu DM, Fu L, Wang H, Sang SF, Chen YF, Hu Q (2012) Optimization and experiment of assessment method for pod shatter resistance in Brassica napus L. Trans Chinese Soc Agric Eng 29(21):19–25. https://doi.org/10.3969/j.issn.1002-6819.2013.21.003
Price JS, Hobson RN, Neale MA, Bruce DM (1996) Seed losses in commercial harvesting of oilseed rape. J Agric Eng Res 65(3):183–191. https://doi.org/10.1006/jaer.1996.0091
Rajani S, Sundaresan V (2002) The Arabidopsis myc/bHLH gene ALCATRAZ enables cell separation in fruit dehiscence. Curr Biol 11(24):1914–1922. https://doi.org/10.1016/S0960-9822(01)00593-0
Riechmann JL, Meyerowitz EM (1997) MADS domain proteins in plant development. Biol Chem 378(10):1079–1101. https://doi.org/10.1515/bchm.1997.378.10.1079
Roeder AHK, Ferrándiz C, Yanofsky MF (2003) The role of the REPLUMLESS homeodomain protein in patterning the Arabidopsis Fruit. Curr Biol Cb 13(18):1630–1635. https://doi.org/10.1016/j.cub.2003.08.027
Romera Branchat M, Ripoll JJ, Yanofsky MF, Pelaz S (2012) The WOX13 homeobox gene promotes replum formation in the Arabidopsis thaliana fruit. Plant J Cell Mol Biol. https://doi.org/10.1111/tpj.12010
Rounsley SD, Ditta GS, Yanofsky MF (1995) Diverse roles for MADS box genes in Arabidopsis development. Plant Cell 7(8):1259–1269. https://doi.org/10.2307/3870100
Sander L, Child RP, Albrechtsen M, Borkhardt B (2001) Analysis of a dehiscence zone endo-polygalacturonase in oilseed rape (Brassica napus) and Arabidopsis thaliana: evidence for roles in cell separation in dehiscence and abscission zones, and in stylar tissues during pollen tube growth. Plant Mol Biol 46(4):469–479. https://doi.org/10.1023/A:1010619002833
Savidge B, Rounsley SD, Yanofsky MF (1995) Temporal relationship between the transcription of two Arabidopsis MADS box genes and the floral organ identity genes. Plant Cell 7(6):721–733. https://doi.org/10.1105/tpc.7.6.721
Silhavy D, Molnar A, Lucioli A, Szittya G, Hornyik C, Tavazza M (2002) A viral protein suppresses RNA silencing and binds silencing-generated, 21- to 25-nucleotide double-stranded RNAs. EMBO J 21(12):3070–3080. https://doi.org/10.1093/emboj/cdf312
Sorefan K, Girin T, Liljegren S, Ljung K, Robles P, Galván-Ampudia C, Offringa R, Friml J, Yanofsky M, Ostergaard L (2009) A regulated auxin minimum is required for seed dispersal in Arabidopsis. Nature 459:583–586. https://doi.org/10.1038/nature07875
Squires TM, Gruwel MLH, Zhou R, Sokhansanj S, Abrams SR, Cutler AJ (2003) Dehydration and dehiscence in siliques of Brassica napus and Brassica rapa. Canadian J Botany-Revue Canadienne Botanique 81(3):248–254. https://doi.org/10.1139/B03-019
Tan XL, Zhang JF, Li Y, Zhang ZY, Jia Z, Song J, Qi CK (2006) Quantitive determination of the strength of rapeseed pod dehiscence. AGRONOMY Crop Physiol/Trans Chinese Soc Agric Eng 22:40–43
Tan XL, Yan SZ, Tan RK, Zhang ZY, Wang Z, Chen J (2014) Characterization and expression of a GDSL-Like Lipase Gene from Brassica napus in Nicotiana benthamiana. Protein J 33(1):18–23. https://doi.org/10.1007/s10930-013-9532-z
Tys J (1985) Evaluation of the mechanical properties of winter rape siliques in respect to their susceptibility to cracking. J Public Adm Policy Res 115:e512-517. https://doi.org/10.1542/peds.2004-1977
van Gelderen K, van Rongen M, Liu A, Otten A, Offringa R (2016) An INDEHISCENT-controlled Auxin response specifies the separation layer in early Arabidopsis fruit. Mol Plant 9(6):857–869. https://doi.org/10.1016/j.molp.2016.03.005
Wen YC, Fu TD, Tu JX (2008) Screening and analysis of resistance to silique shattering in Rape (Brassica napus L.). Acta Agronomica Sinica 34(1):163–166. https://doi.org/10.3724/SP.J.1006.2008.00163
Wen YC, Fu TD, Tu JX, Ma CZ, Shen JX, Zhang SF (2009) Advances in studies of Pod shattering resistance in rapeseed. J Plant Genet Resour 1:140–145
Wielopolska A, Townley H, Moore I, Waterhouse P, Helliwell C (2005) A high throughput inducible RNAi vector for plants. Plant Biotechnol J 3:583–590. https://doi.org/10.1111/j.1467-7652.2005.00149.x
Wu H, Mori A, Jiang XS, Wang YX, Yang M (2006) The INDEHISCENT protein regulates unequal cell divisions in Arabidopsis fruit. Planta 224(4):971–979. https://doi.org/10.1007/s00425-006-0351-8
Xiang Y, Lu YH, Song M, Wang Y, Xu WQ, Wu LT, Wang HC, Ma ZQ (2015) Overexpression of a Triticum aestivum Calreticulin gene (TaCRT1) Improves Salinity Tolerance in Tobacco. PLoS ONE 10(10):e0140591. https://doi.org/10.1371/journal.pone.0140591
Yu YK, Li YL, Ding LN, Sarwar R, Zhao FY, Tan XL (2020) Mechanism and regulation of Silique Dehiscence, which affects oil seed production. Front Plant Sci. https://doi.org/10.3389/fpls.2020.00580
Zheng SQ, Mo XR, Zhu C, Zeng GW (1999) Study on the formation of silique dehiscence susceptabil ity in oilseed rape. J Zhejiang Univ (Ag ric Life Sci) CNKI:SUN:ZJNY.0.1999-05-001
Funding
This work was supported by the National Key R&D Program of China (2016YFD0101900 and 2016YFD0100305).
Author information
Authors and Affiliations
Contributions
XLT, YLL and YKY designed the experiments. YKY, YLL, KMZ, LND, ZW, YHY and JC performed the experiments and analysed the data. YLL and YKY wrote the paper. YLL, YKY, LZX, YML and XLT revised the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Communicated by Baochun Li.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
299_2020_2638_MOESM2_ESM.tif
Fig. S1 The installation of quantitative determination of the fore for silique dehiscence The siliques were glued to a plate, and the replum was paralleled to the plate. One side of L-shaped hook was fixed in the probe of the texture analyser (TAXT2-HD, Stable Micro System, UK), another side hooked the pedicel at the joint point of silique and pedicel. During the test, the plate was held in hands, the probe was moved upwards at the speed of 2 mm/min and the silique was opened. At the same time, the probe recorded the opening strength (TIF 34311 KB)
299_2020_2638_MOESM3_ESM.tif
Fig. S2 Expression pattern of MAN7 homologous genes in B. napus. A total of 7 homologs were obtained from the B. napus genome based on the BLAT search in B. napus genomic database using AtMAN7 protein (NP_201447.1) sequence. The expression level of these genes in different tissues is compared reference to Fragments Per Kilobase per Million (FPKM). The numbers in the table represent the FPKM and the highlighted BnaA07g12590D, which showed the highest expression level among 7 homologs was selected as the target gene in present study. This table is organized according to the BrassicaEDB (https://biodb.swu.edu.cn/brassica/). (TIF 5565 KB)
299_2020_2638_MOESM4_ESM.tif
Fig. S3 Phylogenetic relationship of BnMAN7A07 with other closely related MAN7 proteins. A phylogenetic tree of 6 MAN7 proteins from Brassica napus, Brassica rapa, Raphanus sativus, Sisymbrium officinale and Arabidopsis thaliana was generated in MEGA7 software using the neighbor-joining (NJ) method. A total of 500 boots trap replicates were performed and values are indicated above the branch points (TIF 5646 KB)
299_2020_2638_MOESM5_ESM.tif
Fig. S4 Bioinformatic analyses of BnMAN7A07 protein. a Signal peptide prediction of BnMAN7A07. SignalP-5.0 Server was used to predict the signal peptide of BnMAN7A07 protein. It showed that the cleavage site is between 25 and 26 amino acids with 0.9587 reliability. SP: Signal peptide score; CS: the cleavage site and OTHER: the probability that the sequence does not have any kind of signal peptide. b Subcellular localization prediction of BnMAN7A07. The subcellular localization of BnMAN7A07 protein was predicted by Euk-mPLoc2.0 Server. It predicted BnMAN7A07 localized at extra cell (TIF 12003 KB)
299_2020_2638_MOESM6_ESM.tif
Fig. S5 The standard curve for the xylose content calculation. Different amount of xylose (0, 0.2 mg, 0.4 mg, 0.6 mg, 0.8 mg, 1 mg) was mix with NaAc buffer and DNS. The solution was boiled at 100 ℃ in water bath for 5 min. Add total volume to 25ml with ddH2O and OD540 absorption value was detected by spectrophotometer. The standard curve was performed using Excel (TIF 4639 KB)
299_2020_2638_MOESM7_ESM.tif
Fig. S6 PCR analysis of positive transformed plants using NPT-II-specific primers. The number represent different RNAi lines. +: the plasmid as template, -: ddH2O as template (TIF 4064 KB)
299_2020_2638_MOESM8_ESM.tif
Fig. S7 Time-force curve record exterior forces necessary for silique dehiscence. The pulling force on the silique increased continuously along with the probe moving, and when the silique ripped, the force on the silique rapidly decreased, and the peak of the curve record the max exterior force that was needed for the silique dehiscence (TIF 28881 KB)
299_2020_2638_MOESM9_ESM.tif
Fig. S8 Significance analysis of the force for opening the siliques from different lines. The number represent different siliques form each plant. The significances of the force differences between WT, BnMAN7A07-RNAi line 2 and 3 and are indicated (Student’s t-test, *0.01 < P < 0.05, **** P < 0.0001) (TIF 2387 KB)
Rights and permissions
About this article
Cite this article
Li, YL., Yu, YK., Zhu, KM. et al. Down-regulation of MANNANASE7 gene in Brassica napus L. enhances silique dehiscence-resistance. Plant Cell Rep 40, 361–374 (2021). https://doi.org/10.1007/s00299-020-02638-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-020-02638-5