Abstract
Key message
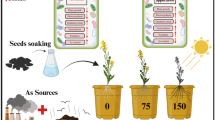
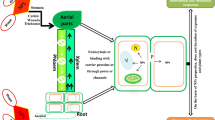
Withania coagulans (L.) Dunal bio-synthesized silver nanoparticles (WcAgNPs) worked as an abiotic elicitor or auto-catalyst that enhanced root regeneration and withanolides production in in-vitro regenerated W. coagulans.
Abstract
Rapid development in the production / consumption of silver nanoparticles (AgNPs) raised serious concern over its effects on the growth of natural plant community. The knowledge related to impact of AgNPs on plant growth and biocompatibility is increasing day by day, but comprehensive mechanism and gaps regarding their impacts on plant health have yet to be addressed. In the present study, we investigated the impact of Withania coagulans biosynthesized AgNPs (WcAgNPs) on in-vitro plant growth and withanolides production. Obtained results showed that the low concentrations of WcAgNPs significantly induced the plant growth by regulating oxidative stress via anti-oxidative defense system. Physiological, morphology and anatomical features also reflected healthy plant growth under low WcAgNPs exposure. While higher concentrations of WcAgNPs have a negative impact on W. coagulans plant growth due to induced lipid peroxidation, ROS accumulation, and root cell death. At lower concentrations, WcAgNPs have shown a positive effect on in-planta withanolides biosynthesis stimulating withanolide A and withaferin A up to 11.15–22.8-fold, respectively. Furthermore, the expression of withanolides biosynthetic genes were also quantified upon WcAgNPs exposure and terpenes biosynthetic genes showed over-expression. Thus, the present study concludes that the lower concentrations of WcAgNPs positively induced plant growth via improved root organogenesis and also have potential to act as an elicitor for withanolides production.







Similar content being viewed by others
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ayala A, Muñoz MF, Argüelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. https://doi.org/10.1155/2014/360438
Azeez L, Adejumo AL, Lateef A, Adebisi SA, Adetoro RO, Adewuyi S, Tijani KO, Olaoye S (2019) Zero-valent silver nanoparticles attenuate Cd and Pb toxicities on Moringa oleifera via immobilization and induction of phytochemicals. Plant Physiol Biochem 139:283–292
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Ann Biochem 44:276–287
Begum S, Zahid A, Khan T, Khan NZ, Ali W (2020) Comparative analysis of the effects of chemically and biologically synthesized silver nanoparticles on biomass accumulation and secondary metabolism in callus cultures of Fagonia indica. Physiol Mol Biol Pla 1–12
Bello-Bello JJ, Chavez-Santoscoy RA, Lecona-Guzman CA, Bogdanchikova N, Salinas-Ruíz J, Gómez-Merino FC, Pestryakov A (2017) Hormetic response by silver nanoparticles on in vitro multiplication of sugarcane (Saccharum spp. Cv. Mex 69–290) using a temporary immersion system. Dose-Response 15(4):1559325817744945
Beyer EMA (1976) potent inhibitor of ethylene action in plants. Plant Physiol 58(3):268–271
Bhat MAMA, Ahmad S, Aslam J, Mujib A (2008) Salinity stress enhances production of solasodine in Solanum nigrum L. Chem Pharm Bull 56(1):17–21
Bundschuh M, Filser J, Lüderwald S, McKee MS, Metreveli G, Schaumann GE, Schulz R, Wagner S (2018) Nanoparticles in the environment: Where do we come from, where do we go to? Environ Sci Eur 30(1):6
Cao Z, Rossi L, Stowers C, Zhang W, Lombardini L, Ma X (2018) The impact of cerium oxide nanoparticles on the physiology of soybean (Glycine max (L.) Merr.) under different soil moisture conditions. Environ Sci Pollut R 25(1):930–939
Chan T, Shimizu Y, Pospíšil P, Nijo N, Fujiwara A, Taninaka Y, Ishikawa T, Hori H, Nanba D, Imai A, Morita N (2012) Quality control of photosystem II: lipid peroxidation accelerates photoinhibition under excessive illumination. PLoS ONE 7(12):e52100
Chen GX, Asada K (1989) Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol 30:987–998
Dutta GS, Agarwal A, Pradhan S (2018) Phytostimulatory effect of silver nanoparticles (AgNPs) on rice seedling growth: An insight from antioxidative enzyme activities and gene expression patterns. Ecotox Environ Safe 161:624–633
Falco WF, Queiroz AM, Fernandes J, Botero ER, Falcão EA, Guimarães FEG, M’Peko JC, Oliveira SL, Colbeck I, Caires ARL (2015) Interaction between chlorophyll and silver nanoparticles: a close analysis of chlorophyll fluorescence quenching. J Photoch Photobio A 299:203–209
Falco WF, Scherer MD, Oliveira SL, Wender H, Colbeck I, Lawson T, Caires AR (2020) Phytotoxicity of silver nanoparticles on Vicia faba: evaluation of particle size effects on photosynthetic performance and leaf gas exchange. Sci Total Environ 701:134816
Fazal H, Abbasi BH, Ahmad N, Ali M (2016) Elicitation of medicinally important antioxidant secondary metabolites with silver and gold nanoparticles in callus cultures of Prunella vulgaris L. Appl Biochem Biotechnol 180(6):1076–1092
Gago J, de Menezes DD, Figueroa CM, Flexas J, Fernie AR, Nikoloski Z (2016) Relationships of leaf net photosynthesis, stomatal conductance, and mesophyll conductance to primary metabolism: a multispecies meta-analysis approach. Plant Physiol 171(1):265–279
Hassiotou F, Renton M, Ludwig M, Evans JR, Veneklaas EJ (2010) Photosynthesis at an extreme end of the leaf trait spectrum: how does it relate to high leaf dry mass per area and associated structural parameters? J Exp Bot 61(11):3015–3028
Hatami M, Naghdi Badi H, Ghorbanpour M (2019) Nano-Elicitation of Secondary Pharmaceutical Metabolites in Plant Cells: A Review. J medi plant 3(71):6–36
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Homaee MB, Ehsanpour AA (2016) Silver nanoparticles and silver ions: Oxidative stress responses and toxicity in potato (Solanum tuberosum L) grown in vitro. Hortic Environ Biotechnol 57(6):544–553
Iavicoli I, Leso V, Fontana L, Calabrese EJ (2018) Nanoparticle exposure and hormetic dose–responses: an update. Int J Mol Sci 19(3):805
Imeh U, Khokhar S (2002) Distribution of conjugated and free phenols in fruits: antioxidant activity and cultivar variations. J Agric Food Chem 50:6301–6306. https://doi.org/10.1021/jf020342j
Jasim B, Thomas R, Mathew J, Radhakrishnan EK (2017) Plant growth and diosgenin enhancement effect of silver nanoparticles in Fenugreek (Trigonella foenum-graecum L.). Saudi Pharm J 25(3):443–447
Kaveh R, Li YS, Ranjbar S, Tehrani R, Brueck CL, Van Aken B (2013) Changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions. Environ Sci Technol 47(18):10637–10644
Khan MIR, Iqbal N, Masood A, Per TS, Khan NA (2013) Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal Behave 8(11):e26374
Kim DH, Gopal J, Sivanesan I (2017) Nanomaterials in plant tissue culture: the disclosed and undisclosed. RSC Adv 7(58):36492–36505
Krishnaraj C, Jagan EG, Ramachandran R, Abirami SM, Mohan N, Kalaichelvan PT (2012) Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) Wettst. plant growth metabolism. Process biochem 47(4):651–658
Lee SH, Jun BH (2019) Silver nanoparticles: synthesis and application for nanomedicine. Int J Mol Sci 20(4):865
León-Silva S, Fernández-Luqueño F, López-Valdez F (2016) Silver Nanoparticles (AgNP) in the environment: a review of potential risks on human and environmental health. Water, Air & Soil Pollut 227(9):306
Mancinelli AL, Yang CH, Lindquist P, Anderson OR, Rabino I (1975) Photocontrol of Anthocyanin synthesis: III. The action of streptomycin on the synthesis of chlorophyll and anthocyanin. Plant Physiol 55:251–257. https://doi.org/10.1104/pp.55.2.251
Maurya R, Akanksha J (2010) Chemistry and pharmacology of Withania coagulans: an Ayurvedic remedy. J Pharm Pharmacol 62(2):153–160
Mhamdi A, Van Breusegem F (2018) Reactive oxygen species in plant development. Development 145(15): dev164376
Mirzajani F, Askari H, Hamzelou S, Farzaneh M, Ghassempour A (2013) Effect of silver nanoparticles on Oryza sativa L. and its rhizosphere bacteria. Ecotoxicol Environ Safe 88:48–54
Mittler R (2017) ROS are good. Trends Plant Sci 22(1):11–19
Mueller NC, Nowack B (2008) Exposure modeling of engineered nanoparticles in the environment. Environ Sci Technol 42:4447–4453
Murashige T, Skoog FA (1962) revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497
Nair PMG, Chung IM (2014a) Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings. Chemosphere 112:105–113
Nair PMG, Chung IM (2014b) Assessment of silver nanoparticle-induced physiological and molecular changes in Arabidopsis thaliana. Environ Sci Pollut R 21(14):8858–8869
Nair PMG, Chung IM (2014c) Impact of copper oxide nanoparticles exposure on Arabidopsis thaliana growth, root system development, root lignification, and molecular level changes. Environ Sci Pollut Res 21(22):12709–12722
Orman-Ligeza B, Parizot B, De Rycke R, Fernandez A, Himschoot E, Van Breusegem F, Bennett MJ, Périlleux C, Beeckman T, Draye X (2016) RBOH-mediated ROS production facilitates lateral root emergence in Arabidopsis. Development 143(18):3328–3339
Pandey N, Meena RP, Rai SK, Pandey-Rai S (2016) In-vitro generation of high artemisinin yielding salt tolerant somaclonal variant and development of SCAR marker in Artemisia annua L. Plant Cell Tissue Organ Cult 127(2):301–314
Patel K, Singh RB, Patel DK (2013) Medicinal significance, pharmacological activities, and analytical aspects of solasodine: a concise report of current scientific literature. J Acute Dis 2(2):92–98
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta Bioenerg 975:384–394
Pulit-Prociak J, Banach M (2016) Silver nanoparticles–a material of the future…? Open Chem 14(1):76–91
Qian HF, Han X, Peng XF, Lu T, Liu WP, Fu ZW (2014) The circadian clock gene regulatory module enantioselectively mediates imazethapyr-induced early flowering in Arabidopsis thaliana. J Plant Physiol 171:92–98
Ramezani M, Asghari S, Gerami M, Ramezani F, Abdolmaleki MK (2019) Effect of Silver Nanoparticle Treatment on the Expression of Key Genes Involved in Glycosides Biosynthetic Pathway in Stevia rebaudiana B. Plant. Sugar Tech 1–10.
Rani PU, Yasur J, Loke KS, Dutta D (2016) Effect of synthetic and biosynthesized silver nanoparticles on growth, physiology and oxidative stress of water hyacinth: Eichhornia crassipes (Mart) Solms. Acta physiol plant 38(2):58
Rao MV, Davis KR (1999) Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: the role of salicylic acid. Plant J 17(6):603–614
Rao S, Shekhawat GS (2016) Phytotoxicity and oxidative stress perspective of two selected nanoparticles in Brassica juncea 3. Biotech 6(2):244
Rathore MS, Shekhawat S, Kaur G, Singh RP, Shekhawat NS (2012) Micropropagation of vegetable rennet (Withania coagulans [Stocks] Dunal)—a critically endangered medicinal plant. J Sustainable Forestry 31(8):727–746
Saratale RG, Saratale GD, Shin HS, Jacob JM, Pugazhendhi A, Bhaisare M, Kumar G (2018) New insights on the green synthesis of metallic nanoparticles using plant and waste biomaterials: current knowledge, their agricultural and environmental applications. Environ Sci Pollut R 25(11):10164–10183
Schaedle M, Bassham JA (1977) Chloroplast glutathione reductase. Plant Physiol 59:1011–1012
Sharma P, Bhatt D, Zaidi MGH, Saradhi PP, Khanna PK, Arora S (2012) Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl Biochem Biotechnol 167(8):2225–2233
Shekhawat GS, Arya V (2009) Biological synthesis of Ag nanoparticles through in vitro cultures of Brassica juncea C. zern. Adv Mater Res 67:295–299
Singh R, Singh DP, Gupta P, Jain P, Mishra T, Kumar A, Dhawan SS, Shirke PA (2019) Nanoparticles alter the withanolide biosynthesis and carbohydrate metabolism in Withania somnifera (Dunal). Ind Crop Prod 127:94–109
Spinoso-Castillo JL, Chavez-Santoscoy RA, Bogdanchikova N, Pérez-Sato JA, Morales-Ramos V, Bello-Bello JJ (2017) Antimicrobial and hormetic effects of silver nanoparticles on in vitro regeneration of vanilla (Vanilla planifolia Jacks. ex Andrews) using a temporary immersion system. Plant Cell Tissue Organ Cult 129(2):195–207
Steinitz B, Barr N, Tabib Y, Vaknin Y, Bernstein N (2010) Control of in vitro rooting and plant development in Corymbia maculata by silver nitrate, silver thiosulfate and thiosulfate ion. Plant Cell Rep 29(11):1315–1323
Strader LC, Beisner ER, Bartel B (2009) Silver ions increase auxin efflux independently of effects on ethylene response. Plant Cell 21:3585–3590
Syu YY, Hung JH, Chen JC, Chuang HW (2014) Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant physiol Biochem 83:57–64
Thiruvengadam M, Gurunathan S, Chung IM (2015) Physiological, metabolic, and transcriptional effects of biologically-synthesized silver nanoparticles in turnip (Brassica rapa ssp. rapa L.). Protoplasma 252(4):1031–1046
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11:1187–1194
Tripathi D, Rai KK, Rai SK, Rai SP (2018) An improved thin cell layer culture system for efficient clonal propagation and in vitro withanolide production in a medicinal plant Withania coagulans Dunal. Ind Crop Prod 119:172–182
Tripathi D, Modi A, Narayan G, Rai SP (2019) Green and cost effective synthesis of silver nanoparticles from endangered medicinal plant Withania coagulans and their potential biomedical properties. Mater Sci Eng: C 1001:52–164
Usman M, Farooq M, Wakeel A, Nawaz A, Cheema SA, ur Rehman H, Ashraf I, Sanaullah M (2020) Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci Total Environ 137778.
Vinković T, Novák O, Strnad M, Goessler W, Jurašin DD, Parađiković N, Vrček IV (2017) Cytokinin response in pepper plants (Capsicum annuum L.) exposed to silver nanoparticles. Environ Res 156:10–18
Vishwakarma K, Upadhyay N, Singh J, Liu S, Singh VP, Prasad SM, Chauhan DK, Tripathi DK, Sharma S (2017) Differential Phytotoxic Impact of Plant Mediated Silver Nanoparticles (AgNPs) and Silver Nitrate (AgNO3) on Brassica sp. Front Plant Sci 8:1501
Wang P, Lombi E, Zhao FJ, Kopittke PM (2016) Nanotechnology: a new opportunity in plant sciences. Trends plant sci 21(8):699–712
Wang XP, Li QQ, Pei ZM, Wang SC (2018) Effects of zinc oxide nanoparticles on the growth, photosynthetic traits, and antioxidative enzymes in tomato plants. Biol Plant 62(4):801–808
Yan A, Chen Z (2019) Impacts of Silver Nanoparticles on Plants: A Focus on the Phytotoxicity and Underlying Mechanism. Int J Mol Sci 20(5):1003
Yin L, Cheng Y, Espinasse B, Colman BP, Auffan M, Wiesner M, Rose J, Liu J, Bernhardt ES (2011) More than the ions: the effects of silver nanoparticles on Lolium multiflorum. Environ Sci Technol 45(6):2360–2367
Yuan J, Chen Y, Li H, Lu J, Zhao H, Liu M, Nechitaylo GS, Glushchenko NN (2018) New insights into the cellular responses to iron nanoparticles in Capsicum annuum. Sci Rep 8(1):3228
Yue L, Ma C, Zhan X, White JC, Xing B (2017) Molecular mechanisms of maize seedling response to La2O3 NP exposure: water uptake, aquaporin gene expression and signal transduction. Environ Sci Nano 4(4):843–855
Zafar H, Ali A, Ali JS, Haq IU, Zia M (2016) Effect of ZnO nanoparticles on Brassica nigra seedlings and stem explants: growth dynamics and antioxidative response. Front Plant Sci 7:535
Zhang B, Zheng LP, Yi Li W, Wen Wang J (2013) Stimulation of artemisinin production in Artemisia annua hairy roots by Ag-SiO2 core-shell nanoparticles. Curr Nanosci 9(3):363–370
Zuverza-Mena N, Armendariz R, Peralta-Videa JR, Gardea-Torresdey JL (2016) Effects of silver nanoparticles on radish sprouts: root growth reduction and modifications in the nutritional value. Front Plant Sci 7:90
Acknowledgements
All the authors are highly obliged towards Padma Shri awarded Prof. O. N. Srivastava, Department of Physics, BHU-Varanasi for providing SEM facility. We are grateful to CAS- Botany (BHU), DST-PURSE and DST-FIST for providing funds to the Department of Botany, Institute of Science, Banaras Hindu University- Varanasi, India. We are also very thankful to Interdisciplinary School of Life Sciences (ISLS) for providing ICP-MS, confocal and fluorescence microscope facilities. We are highly thankful to AIRF- JNU (New Delhi) for LC-MS analysis. Deepika Tripathi is thankful to CSIR/UGC- New Delhi, India for providing financial support throughout the presented work.
Author information
Authors and Affiliations
Contributions
DT and SPR designed the experimental work. All experiments were performed by DT. KKR helped during gene expression analysis and Heat-map preparation. DT analyzed the data and wrote the manuscript. SPR approved the final manuscript after editorial help.
Corresponding author
Ethics declarations
Conflict of interest
Authors are declaring no conflicts of interest.
Additional information
Communicated by Kan Wang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tripathi, D., Rai, K.K. & Pandey-Rai, S. Impact of green synthesized WcAgNPs on in-vitro plant regeneration and withanolides production by inducing key biosynthetic genes in Withania coagulans. Plant Cell Rep 40, 283–299 (2021). https://doi.org/10.1007/s00299-020-02630-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-020-02630-z