Abstract
Key message
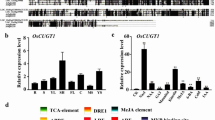
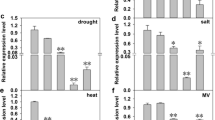
RTD1 encodes a homogentisate phytyltransferase catalyzing a key step in rice tocopherol biosynthesis, confers cold tolerance and regulates rice development by affecting the accumulation of DELLA protein SLENDER RICE1.
Abstract
Tocopherols are one of the most important lipid-soluble antioxidants having indispensable roles in living organisms. The physiological functions of tocopherols have been comprehensively characterized in animals and artificial membranes. However, genetic and molecular functions of tocopherols in plants are less understood. This study aimed to isolate a tocopherol-deficient mutant rtd1 in rice. The rtd1 mutant showed overall growth retardation throughout the growth period. Most of the agronomic traits were impaired in rtd1. Map-based cloning revealed that the RTD1 gene encoded a homogentisate phytyltransferase, a key enzyme catalyzing the committed step in tocopherol biosynthesis. RTD1 was preferentially expressed in green leafy tissues, and the protein was located in chloroplasts. Cold tolerance was found to be reduced in rtd1. The cold-related C-repeat-binding factor (CBF)/dehydration-responsive element-binding protein 1 (DREB1) genes were significantly upregulated in rtd1 under natural growth conditions. Moreover, rtd1 exhibited a reduced response to gibberellin (GA).The transcript and protein levels of DELLA protein-coding gene SLENDER RICE 1 (SLR1) in rice was increased in rtd1. However, the GA content was not changed, suggesting a transcriptional, not posttranslational, regulation of SLR1. These findings implied that tocopherols play important roles in regulating rice growth and development.







Similar content being viewed by others
Abbreviations
- CBF:
-
C-repeat-binding factor
- DMPBQ:
-
2,3-Dimethyl-5-phytyl-1,4-benzoquinone
- DREB1:
-
Dehydration-responsive element-binding protein 1
- GAs:
-
Gibberellins
- GFP:
-
Green fluorescent protein
- GGDP:
-
Geranylgeranyl diphosphate
- GID1:
-
Gibberellin-insensitive dwarf1
- GID2:
-
Gibberellin-insensitive dwarf2
- HGA:
-
Homogentisic acid
- HPLC:
-
High-performance liquid chromatography
- HPP:
-
p-Hydroxyphenylpyruvate
- HPT:
-
Homogentisate phytyltransferase
- MPBQ:
-
2-Methyl-6-phytylbenzoquinol
- PDP:
-
Phytyl diphosphate
- PUFAs:
-
Polyunsaturated fatty acids
- RNAi:
-
RNA interference
- SLR1:
-
SLENDER RICE1
References
Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, Harberd NP (2006) Integration of plant responses to environmentally activated phytohormonal signals. Science 311:91–94
Achard P, Gong F, Cheminant S, Alioua M, Hedden P, Genschik P (2008) The cold-inducible CBF1 factor-dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell 20:2117–2129
Bergmüller E, Porfirova S, Dörmann P (2003) Characterization of an Arabidopsis, mutant deficient in γ-tocopherol methyltransferase. Plant Mol Biol 52:1181–1190
Bramley PM, Elmadfa I, Kafatos A, Kelly FJ, Manios Y, Roxborough HE, Schuch W, Sheehy PJA, Wagner KH (2000) Vitamin E. J Sci Food Agr 80:913–938
Chaudhary N, Khurana P (2009) Vitamin E biosynthesis genes in rice: molecular characterization, expression profiling and comparative phylogenetic analysis. Plant Sci 177:479–491
Chen S, Tao L, Zeng L, Vega-Sanchez ME, Umemura K, Wang GL (2006) A highly efficient transient protoplast system for analyzing defence gene expression and protein–protein interactions in rice. Mol Plant Pathol 7:417–427
Cheng Z, Sattler S, Maeda H, Sakuragi Y, Bryant DA, DellaPenna D (2003) Highly divergent methyltransferases catalyze a conserved reaction in tocopherol and plastoquinone synthesis in cyanobacteria and photosynthetic eukaryotes. Plant Cell 15:2343–2356
Claeys H, Skirycz A, Maleux K, Inzé D (2012) DELLA signaling mediates stress-induced cell differentiation in Arabidopsis leaves through modulation of anaphase-promoting complex/cyclosome activity. Plant Physiol 159:739–747
Collakova E, DellaPenna D (2003) Homogentisate phytyltransferase activity is limiting for tocopherol biosynthesis in Arabidopsis. Plant Physiol 131:632–642
Cossins AR (1994) Temperature adaptation of biological membranes. Portland, London
DellaPenna D, Pogson BJ (2006) Vitamin synthesis in plants: tocopherols and carotenoids. Annu Rev Plant Biol 57:711–738
Feng S, Martinez C, Gusmaroli G, Wang Y, Zhou J, Wang F, Chen L, Yu L, Iglesias-Pedraz JM, Kircher S, Schäfer E, Fu X, Fan LM, Deng XW (2008) Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 451:475–479
Fryer MJ (2006) The antioxidant effects of thylakoid vitamin E (α-tocopherol). Plant Cell Environ 15:381–392
Gilmour SJ, Zarka DG, Stockinger EJ, Salazar MP, Houghton JM, Thomashow MF (1998) Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J 16:433–442
Grusak MA, DellaPenna D (1999) Improving the nutrient composition of plants to enhance human nutrition and health. Annu Rev Plant Physiol Plant Mol Biol 50:133–161
Havaux M, Eymery F, Porfirova S, Rey P, Dörmann P (2005) Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell 17:3451–3469
Hirano K, Aya K, Hobo T, Sakakibara H, Kojima M, Shim RA, Hasegawa Y, Ueguchi-Tanaka M, Matsuoka M (2008) Comprehensive transcriptome analysis of phytohormone biosynthesis and signaling genes in microspore/pollen and tapetum of rice. Plant Cell Physiol 49:1429–1450
Ikeda A, Ueguchi-Tanaka M, Sonoda Y, Kitano H, Koshioka M, Futsuhara Y, Matsuoka M, Yamaguchi J (2001) Slender rice, a constitutive gibberellin response mutant, is caused by a null mutation of the SLR1 gene, an ortholog of the height-regulating gene GAI/RGA/RHT/D8. Plant Cell 13:999–1010
Ito Y, Katsura K, Maruyama K, Taji T, Kobayashi M, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2006) Functional analysis of rice DREB1/CBF-type transcription factors involved in cold-responsive gene expression in transgenic rice. Plant Cell Physiol 47:141–153
Jaglo-Ottosen KR, Gilmour SJ, Zarka DG, Schabenberger O, Thomashow MF (1998) Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 280:104–106
Jiang Q, Elsonschwab I, Courtemanche C, Ames BN (2000) γ-tocopherol and its major metabolite, in contrast to α-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci USA 97:11494–11499
Kamal-Eldin A, Appelqvist LA (1996) The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 31:671–701
Kempná P, Reiter E, Arock M, Azzi A, Zingg JM (2004) Inhibition of HMC-1 mast cell proliferation by vitamin E: involvement of the protein kinase B pathway. J Biol Chem 279:50700–50709
Kobayashi M, Yamaguchi I, Murofushi N, Ota Y, Takahashi N (2006) Fluctuation and localization of endogenous gibberellins in rice. Agri Biol Chem 52:1189–1194
Li H, Jiang L, Youn JH, Sun W, Cheng Z, Jin T, Ma X, Guo X, Wang J, Zhang X, Wu F, Wu C, Kim SK, Wan J (2013) A comprehensive genetic study reveals a crucial role of CYP90D2/D2 in regulating plant architecture in rice (Oryza sativa). New Phytol 200:1076–1088
Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10:1391–1406
Lyons J (1973) Chilling injury in plants. Annu Rev Plant Physiol 24:445–466
Maeda H, Song W, Sage TL, DellaPenna D (2006) Tocopherols play a crucial role in low-temperature adaptation and phloem loading in Arabidopsis. Plant Cell 18:2710–2732
Maeda H, Sage TL, Isaac G et al (2008) Tocopherols modulate extraplastidic polyunsaturated fatty acid metabolism in Arabidopsis at low temperature. Plant Cell 20:452–470
Magome H, Yamaguchi S, Hanada A, Kamiya Y, Oda K (2008) The DDF1 transcriptional activator upregulates expression of a gibberellin-deactivating gene, GA2ox7, under high-salinity stress in Arabidopsis. Plant J 56:613–626
Magome H, Nomura T, Hanada A, Takeda-Kamiya N, Ohnishi T, Shinma Y, Katsumata T, Kawaide H, Kamiya Y, Yamaguchi S (2013) CYP714B1 and CYP714B2 encode gibberellin 13-oxidases that reduce gibberellin activity in rice. Proc Natl Acad Sci USA 110:1947–1952
Mao D, Chen C (2012) Colinearity and similar expression pattern of rice DREB1s reveal their functional conservation in the cold-responsive pathway. PLoS One 7:e47275
Miquel M, Jr JD, Dooner H et al (1993) Arabidopsis requires polyunsaturated lipids for low-temperature survival. Proc Natl Acad Sci USA 90:6208–6212
Munné-Bosch S (2005) Linking tocopherols with cellular signaling in plants. New Phytol 166:363–366
Munné-Bosch S, Falk J (2004) New insights into the function of tocopherols in plants. Planta 218:323–326
Munné-bosch S, Weiler EW, Alegre L, Müller M, Düchting P, Falk J (2007) Alpha-tocopherol may influence cellular signaling by modulating jasmonic acid levels in plants. Planta 225:681–691
Nishimura A, Aichi I, Matsuoka M (2006) A protocol for Agrobacterium-mediated transformation in rice. Nat Protoc 1:2796–2802
Norris S, Shen X, DellaPenna D (1998) Complementation of the Arabidopsis pds1 mutation with the gene encoding p-hydroxyphenylpyruvate dioxygenase. Plant Physiol 117:1317–1323
Porfirova S, Bergmuller E, Tropf S, Lemke R, Dormann P (2002) Isolation of an Arabidopsis mutant lacking vitamin E and identification of a cyclase essential for all tocopherol biosynthesis. Proc Natl Acad Sci USA 99:12495–12500
Richards DE, King KE, Ait-Ali T, Harberd NP (2001) How gibberellin regulates plant growth and development: a molecular genetic analysis of gibberellin signaling. Annu Rev Plant Physiol Plant Mol Biol 52:67–88
Rimbach G, Minihane AM, Majewicz J, Fischer A, Pallauf J, Virgli F, Weinberg PD (2002) Regulation of cell signalling by vitamin E. Proc Nutr Soc 61:415–425
Russell NJ (1984) Mechanisms of thermal adaptation in bacteria: blueprints for survival. Trends Biochem Sci 9:108–112
Russin WA, Evert RF, Vanderveer PJ, Sharkey TD, Briggs SP (1996) Modification of a specific class of plasmodesmata and loss of sucrose export ability in the sucrose export defective1 maize mutant. Plant Cell 8:645–658
Sakuma Y, Liu Q, Dubouzet JG, Abe H, Shinozaki K, Yamaguchi-Shinozaki K (2002) DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem Biophys Res Commun 290:998–1009
Sanguinetti CJ, Dias NE, Simpson AJ (1994) Rapid silver staining and recovery of PCR products separated on polyacrylamide gels. Biotechniques 17:914–921
Sasaki A, Itoh H, Gomi K, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Jeong DH, An G, Kitano H, Ashikari M, Matsuoka M (2003) Accumulation of phosphorylated repressor for gibberellins signaling in an F-box mutant. Science 299:1896–1898
Sattler SE, Cahoon EB, Coughlan SJ, DellaPenna D (2003) Characterization of tocopherol cyclases from higher plants and cyanobacteria. evolutionary implications for tocopherol synthesis and function. Plant Physiol 132:2184–2195
Sattler SE, Gilliland LU, Magallanes-Lundback M, Pollard M, DellaPenna D (2004) Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell 16:1419–1432
Shinwari ZK, Nakashima K, Miura S, Kasuga M, Seki M, Yamaguchi-Shinozaki K, Shinozaki K (1998) An Arabidopsis gene family encoding DRE/CRT binding proteins involved in low-temperature-responsive gene expression. Biochem Biophys Res Commun 250:161–170
Soll J (1987) α-tocopherol and plastoquinone synthesis in chloroplast membranes. Method Enzymol 148:383–392
Stockinger EJ, Gilmour SJ, Thomashow MF (1997) Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-Repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci USA 94:1035–1040
Sun TP, Gubler F (2004) Molecular mechanism of gibberellin signaling in plants. Annu Rev Plant Biol 55:197–223
Ueguchi-Tanaka M, Ashikari M, Nakajima M, Itoh H, Katoh E, Kobayashi M, Chow TY, Hsing YI, Kitano H, Yamaguchi I, Matsuoka M (2005) GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 437:693–698
Valentin HE, Lincoln K, Moshiri F, Jensen PK, Qi Q, Venkatesh TV, Karunanandaa B, Baszis SR, Norris SR, Savidge B, Gruys KJ, Last RL (2006) The Arabidopsis vitamin E pathway gene5-1 mutant reveals a critical role for phytol kinase in seed tocopherol biosynthesis. Plant Cell 18:212–224
Vidi PA, Kanwischer M, Baginsky S, Austin JR, Csucs G, Dörmann P, Kessler F, Bréhélin C (2006) Tocopherol cyclase (VTE1) localization and vitamin E accumulation in chloroplast plastoglobule lipoprotein particles. J Biol Chem 281:11225–11234
Vom DK, Hölzl G, Plohmann C, Eisenhut M, Abraham M, Weber AP, Hanson AD, Dörmann P (2015) Remobilization of phytol from chlorophyll degradation is essential for tocopherol synthesis and growth of Arabidopsis. Plant Cell 27:2846–2859
Wang X, Quinn PJ (2000) The location and function of vitamin E in membranes (Review). Mol Membr Biol 17:143–156
Wang D, Wang Y, Long W, Niu M, Zhao Z, Teng X, Zhu X, Zhu J, Hao Y, Wang Y, Liu Y, Jiang L, Wang Y, Wan J (2017) SGD1, a key enzyme in tocopherol biosynthesis, is essential for plant development and cold tolerance in rice. Plant Sci 260:90–100
Yang W, Cahoon RE, Hunter SC, Zhang C, Han J, Borgschulte T, Cahoon EB (2011) Vitamin E biosynthesis: functional characterization of the monocot homogentisate geranylgeranyl transferase. Plant J 65:206–217
Yoshida S, Forno DA, Cock JH (1976) Laboratory manual for physiological studies of rice. International Rice Research Institute, Los Baños
Zhang Y, Bian X, Zhang S, Ling J, Wang Y, Wei X, Fang X (2016) Identification of a novel gain-of-function mutant allele slr1-d5, of rice DELLA protein. J Integr Agr 15:1441–1448
Zhao Z, Zhang Y, Liu X, Zhang X, Liu S, Yu X, Ren Y, Zheng X, Zhou K, Jiang L, Guo X, Gai Y, Wu C, Zhai H, Wang H, Wan J (2013) A role for a dioxygenase in auxin metabolism and reproductive development in rice. Dev Cell 27:113–122
Zhou S, Wang Y, Li W, Zhao Z, Ren Y, Wang Y, Gu S, Lin Q, Wang D, Jiang L, Su N, Zhang X, Liu L, Cheng Z, Lei C, Wang J, Guo X, Wu F, Ikehashi H, Wang H, Wan J (2011) Pollen semi-sterility1 encodes a kinesin-1-like protein important for male meiosis, anther dehiscence, and fertility in rice. Plant Cell 23:111–129
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31401036), the Natural Science Foundation of Jiangsu Province, China (BK20161308), the Jiangsu Independent Innovation Project, China (CX(14)5005), and the Basal Research Fund of Jiangsu Academy of Agricultural Sciences, China (ZX(15)4015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by Da-Bing Zhang.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Y., Liu, K., Zhu, X. et al. Rice tocopherol deficiency 1 encodes a homogentisate phytyltransferase essential for tocopherol biosynthesis and plant development in rice. Plant Cell Rep 37, 775–787 (2018). https://doi.org/10.1007/s00299-018-2266-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-018-2266-9