Abstract
Key message
Over-expression of SlJA2 decreased the accumulation of SA, which resulted in significant physiological and gene expression changes in transgenic tobacco plants, leading to the decreased heat tolerance of transgenic tobacco.
Abstract
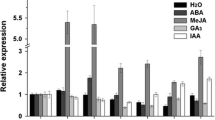
NAC family, the largest transcription factors in plants, responses to different environmental stimuli. Here, we isolated a typical NAC transcription factor (SlJA2) from tomato and got transgenic tobacco with SlJA2 over-expression. Expression of SlJA2 was induced by heat stress (42 °C), chilling stress (4 °C), drought stress, osmotic stress, abscisic acid, and salicylic acid. Over-expression of SlJA2 decreased the accumulation of salicylic acid by regulating expression of salicylic acid degradation gene under heat stress. Compared to WT plants, stomatal apertures and water loss increased in transgenic plants, and the damage of photosynthetic apparatus and chlorophyll breakdown were more serious in transgenic plants under heat stress. Meanwhile, more H2O2 and O2 ·− were accumulated transgenic plants and proline synthesis was restricted, which resulted in more serious oxidative damage compared to WT. qRT-PCR analysis showed that over-expression of SlJA2 could down-regulate genes involved in reactive oxygen species scavenging, proline biosynthesis, and response to heat stress. All the above results indicated that SlJA2 may be a negative regulator responded to plant’s heat tolerance. Thus, this study provides new insight into roles of NAC family member in plant response to abiotic stress.










Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- AbA:
-
Aureobasidin A
- ABRE:
-
ABA-responsive elements
- APX:
-
Ascorbate peroxidase
- CaMV 35S:
-
Cauliflower mosaic virus 35S
- CAT:
-
Catalase
- DAB:
-
3,3′-Diaminobenzidine
- GFP:
-
Green fluorescent protein
- GUS:
-
Beta-glucuronidase
- H2O2 :
-
Hydrogen peroxide
- HPLC–MS/MS:
-
High-performance liquid chromatography tandem mass spectrometry
- LEA:
-
Late embryogenesis abundant proteins
- MDA:
-
Malondialdehyde
- MV:
-
Methyl viologen
- NADPH oxidase:
-
Nicotinamide adenine dinucleotide phosphate oxidase
- NBT:
-
Nitroblue tetrazolium
- O2 ·− :
-
Superoxide anion radical
- OE:
-
Over-expression
- PEG:
-
Polyethylene glycol
- Pn:
-
Net photosynthetic rate
- PSII:
-
Photosystem II
- REL:
-
Relative electrolyte leakage
- ROS:
-
Reactive oxygen species
- qRT-PCR:
-
Real-time quantitative PCR
- RWC:
-
Relative water content
- SA:
-
Salicylic acid
- SlJA2:
-
Solanum lycopersicum jasmonic acid 2
- TFs:
-
Transcription factors
- TR:
-
Transcription regulatory
- WT:
-
Wild-type
References
Acharya BR, Assmann SM (2009) Hormone interactions in stomatal function. Plant Mol Biol 69:451–462
Alia, Saradhi PP, Mohanty P (1997) Involvement of proline in protecting thylakoid membranes against free radical-induced photodamage. J Photochem Photobiol 38:253–257
Clarke SM, Mur LA, Wood JE, Scott IM (2004) Salicylic acid dependent signaling promotes basal thermotolerance but is not essential for acquired thermotolerance in Arabidopsis thaliana. Plant J 38:432–447
Dat JF, Lopez-Delgado H, Foyer CH, Scott IM (2000) Effects of salicylic acid on oxidative stress and thermo tolerance in tobacco. J Plant Physiol 156:659–665
Du MM, Zhai QZ, Deng L, Li SY, Li HS, Yan LH, Huang Z, Wang B, Li CY (2014) Closely related NAC transcription factors of tomato differentially regulate stomatal closure and reopening during pathogen attack. Plant Cell 26(7):3167–3184
Ernst HA, Olsen AN, Larsen S, Lo Leggio L (2004) Structure of the conserved domain of ANAC, a member of the NAC family of transcription factors. EMBO Rep 5:297–303
Fang YJ, You J, Xie KB, Xie WB, Xiong LZ (2008) Systematic sequence analysis and identification of tissue-specific or stress-responsive genes of NAC transcription factor family in rice. Mol Genet Genom 280:535–546
Fang YJ, Liao KF, Du H, Xu Y, Song HZ, Li XH, Xiong LZ (2015) A stress-responsive NAC transcription factor SNAC3 confers heat and drought tolerance through modulation of reactive oxygen species in rice. J Exp Bot 66(21):6803–6817
Feng HL, Ma NN, Meng X, Zhang S, Wang JR, Chai S, Meng QW (2013) A novel tomato MYC-type ICE1-like transcription factor, SlICE1a, confers cold, osmoticand salt tolerance in transgenic tobacco. Plant Physiol Biochem 73:309–320
Fujita M, Fujita Y, Maruyama K, Seki M, Hiratsu K, Ohme-Takagi M, Tran LS, Yamaguchi-Shinozaki K, Shinozaki K (2004) A dehydration induced NAC protein, RD26, is involved in a novel ABA-dependent stress-signaling pathway. Plant J 39:863–887
Giacomelli L, Masi A, Ripoll DR, Lee MJ, van Wijk KJ (2007) Arabidopsis thaliana deficient in two chloroplast ascorbate peroxidases shows accelerated light-induced necrosis when levels of cellular ascorbate are low. Plant Mol Biol 65:627–644
Jensen MK, Kjaersgaard T, Nielsen MM, Galberg P, Petersen K, O’Shea C, Skriver K (2010) The Arabidopsis thaliana NAC transcription factor family: structure-function relationships and determinants of ANAC019 stress signalling. Biochem J 426:183–196
Jiang M, Zhang J (2001) Effect of abscisic acid on active oxygen species, antioxidative defense system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol 42:1265–1273
Khan MI, Fatma M, Per TS, Anjum NA, Khan NA (2015) Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci 6:1–17
Khokon MAR, Hossain MA, Munemasa S, Uraji M, Nakamura Y, Mori IC, Murata Y (2010a) Yeast elicitor-induced stomatal closure and peroxidase-mediated ROS production in Arabidopsis. Plant Cell Physiol 51:1915–1921
Khokon MAR, Okuma E, Hossain MA, Munemasa S, Uraji M, Nakamura Y, Mori IC, Murata Y (2010b) Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant Cell Environ 34:434–443
Kjaersgaard T, Michael KJ, Michael WC, Per G, Birthe BK, Karen S (2011) Senescence-associated barley NAC (NAM, ATAF1, 2, CUC) transcription factor interacts with radical-induced cell death 1 through a disordered regulatory domain. J Biol Chem 286:35418–35429
Kong FY, Deng YS, Zhou B, Wang GD, Wang Y, Meng QW (2014) A chloroplast-targeted DnaJ protein contributes to maintenance of photosystem II under chilling stress. J Exp Bot 65:143–158
Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22:2623–2633
Li JT, Yu G, Sun XH, Zhang XH, Liu JL, Pan HY (2016) AcEBP1, an ErbB3-Binding Protein (EBP1) from halophyte Atriplex canescens, negatively regulates cell growth and stress responses in Arabidopsis. Plant Sci 248:64–74
Ma NN, Zuo YQ, Liang XQ, Yin B, Wang GD, Meng QW (2013) The multiple stress-responsive transcription factor SlNAC1 improves the chilling tolerance of tomato. Physiol Plant 149:474–486
Manthe B, Schulz M, Schnable H (1992) Effects of salicylic acid on growth and stomatal movements on Vicia faba L.: evidence for salicylic acid metabolism. J Chem Ecol 18:1525–1539
Misra N, Saxena P (2009) Effect of salicylic acid on proline metabolism in lentil grown under salinity stress. Plant Sci 177:181–189
Mori IC, Pinontoan R, Kawano T, Muto S (2001) Involvement of superoxide generation in salicylic acid-induced stomatal closure in Vicia faba. Plant Cell Physiol 42:1383–1388
Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J, Morris P, Ribeiro D, Wilson I (2008) Nitric oxide, stomatal closure, and abiotic stress. J Exp Bot 59:165–176
Olsen AN, Ernst HA, Leggio LL, Skriver K (2005) NAC transcription factors: Structurally distinct, functionally diverse. Trends Plant Sci 10:79–87
Rao MV, Davis KR (1999) Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: the role of salicylic acid. Plant J 17:603–614
Raskin I (1992) Role of salicylic acid in plants. Annu Rev Plant Physiol Plant Mol Biol 43:439–463
Seo PJ, Kim SG, Park CM (2008) Membrane-bound transcription factors in plants. Trends Plant Sci 13:550–556
Shan DP, Huang JG, Yang YT, Guo YH, Wu CA, Yang GD, Gao Z, Zheng CC (2007) Cotton GhDREB1 increases plant tolerance to low temperature and is negatively regulated by gibberellic acid. New Phytol 176:70–81
Shi QH, Bao ZY, Zhu ZJ, Ying QS, Qian QQ (2006) Effects of different treatments of salicylic acid on heat tolerance, chlorophyll fluorescence, and antioxidant enzyme activity in seedlings of Cucumis sativa L. Plant Growth Regul 48:127–135
Thomine S, Lelièvre F, Debarbieux E, Schroeder JI, Barbier-Brygoo H (2003) AtNRAMP3, a multispecific vacuolar metal transporter involved in plant responses to iron deficiency. Plant J 34:685–695
Underwood W, Melotto M, He SY (2007) Role of plant stomata in bacterial invasion. Cell Microbiol 9(7):1621–1629
Uzunova AN, Popova LP (2000) Effect of salicylic acid on leaf anatomy and chloroplast ultrastructure of barley plants. Photosynthetica 38:243–250
Wang LJ, Li SH (2006) Salicylic acid-induced heat or cold tolerance in relation to Ca2+ homeostasis and antioxidant systems in young grape plants. Plant Sci 170:685–694
Wang AG, Luo GH, (1990) Quantitative relation between the reaction of hydroxylamine and superoxide anion radicals in plants. Plant Physiol Commun 6:55–57
Xu ZY, Kim SY, Hyeon do Y, Kim DH, Dong T, Park Y, Jin JB, Joo SH, Kim SK, Hong JC, Hwang D, Hwang I (2013) The Arabidopsis NAC transcription factor ANAC096 cooperates with bZIP-type transcription tactors in dehydration and osmotic stress responses. Plant Cell 25:4708–4724
Yang SD, Seo PJ, Yoon HK, Park CM (2011) The Arabidopsis NAC transcription factor VNI2 integrates abscisic acid signals into leaf senescence via the COR/RD genes. Plant Cell 23:2155–2168
Zhong R, Lee CH, Ye ZH (2010) Global analysis of direct targets of secondary wall NAC master switches in Arabidopsis. Mol Plant 3:1087–1103
Zhou ZS, Guo K, Elbaz AA, Yang ZM (2009) Salicylic acid alleviates mercury toxicity by preventing oxidative stress in roots of Medicago sativa. Environ Exp Bot 65:27–34
Zhu JK, Hasegawa PM, Bressan RA (1997) Molecular aspects of osmotic stress in plants. Crit Rev Plant Sci 16:253–277
Zong XJ, Li DP, Gu LK, Li DQ, Liu LX, Hu XL (2009) Abscisic acid and hydrogen peroxide induce a novel maize group C MAP kinase gene, ZmMPK7, which is responsible for the removal of reactive oxygen species. Planta 229:485–495
Acknowledgements
This work was supported by the State Key Basic Research and Development Plan of China (2015CB150105), the Natural Science Foundation of China (31171474, 31371553), and the Doctor Foundation of Shandong (2014BSB01031).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Chun-Hai Dong.
Z.-M. Liu and M.-M. Yue contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, ZM., Yue, MM., Yang, DY. et al. Over-expression of SlJA2 decreased heat tolerance of transgenic tobacco plants via salicylic acid pathway. Plant Cell Rep 36, 529–542 (2017). https://doi.org/10.1007/s00299-017-2100-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-017-2100-9