Abstract
Key message
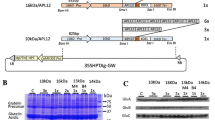
Mouse TGF-β highly accumulated by expressing as a secretory homodimeric protein in transgenic rice endosperm. It was tightly deposited in ER-derived PBs by interaction with cysteine-rich prolamins.
Abstract
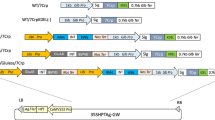
TGF-β is one of the key players involved in the induction and maintenance of mucosal immune tolerance to dietary proteins through the induction of regulatory T cells. In order to utilize rice-based TGF-β as a tool to promote oral immune tolerance induction, high production of TGF-β is essentially required. When the codon-optimized mTGF-β was expressed as a secretory protein by ligating an N-terminal signal peptide and C-terminal KDEL ER retention signal under the control of the endosperm-specific rice storage protein glutelin GluB-1 promoter, accumulation level was low in stable transgenic rice seeds. Then, to increase the accumulation level of mTGF-β, it was expressed as fusion proteins by inserting into the C terminus of acidic subunit of glutelin GluA and the variable region of 26 kDa globulin. When fused with the glutelin, it could accumulate well as visible bands by CBB staining gel, but not for the 26 kDa globulin. Unexpectedly, expression of homodimeric mTGF-β linked by a 6×Gly1×Ser linker as secretory protein resulted in higher level of accumulation. This expression level was further enhanced by reduction of some endogenous prolamins by RNA interference. The monomeric and dimeric mTGF-βs were deposited in ER-derived PBs containing prolamins. When highly produced in rice seed, it is notable that most of ER-derived PBs were distorted and granulated. Step-wise extraction of storage proteins from rice seeds suggested that the mTGF-β strongly interacted with cysteine-rich prolamins via disulfide bonds. This result was also supported by the finding that reducing agent was absolutely required for mTGF-β extraction.








Similar content being viewed by others
Abbreviations
- BiP:
-
Binding protein
- CHO cell:
-
Chinese hamster ovary cell
- CTAB:
-
Cetyl trimethyl ammonium bromide
- Cys-rich prolamins:
-
Cysteine-rich prolamins
- DTT:
-
Dithiothreitol
- ER:
-
Endoplasmic reticulum
- GALT:
-
Gut-associated lymphoid tissue
- GFP:
-
Green fluorescent protein
- PB:
-
Protein body
- 2-MER:
-
2-Mercaptoethanol
- SDS-PAGE:
-
SDS-polyacrylamide gel electrophoresis
- TGF-β:
-
Transforming growth factor
References
Cabanos C, Kato N, Amari Y, Fujiwara K, Ohno T, Shimizu K, Goto T, Shimada M, Kuroda M, Masuda T, Takaiwa F, Utsumi S, Nagaoka S, Maruyama N (2012) Development of a novel transgenic rice with hypocholesterolemic activity via high-level accumulation of the α’ subunit of soybean β-conglycinin. Transgenic Res 23:609–620
Conley AJ, Joensuu JJ, Richman A, Menassa R (2011) Protein body-inducing fusions for high-level production and purification of recombinant proteins in plants. Plant Biteochnol J 9:419–433
Da Cunha NB, Vianna GR, Lima TA, Rech E (2014) Molecular farming of human cytokines and blood products from plants: challenges in biosynthesis and detection of plant-produced recombinant proteins. Biotechnol J 9:39–50
Derynck R, Jarrett J, Chen E, Eaton D, Bell J, Assoian R, Roberts A, Sporn M, Goeddel D (1985) Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature 316:701–715
Desai PN, Shrivastava N, Padh H (2010) Production of heterologous proteins in plants: strategies for optimal expression. Biotechnol Adv 28:427–435
Fujiwara Y, Yang L, Takaiwa F, Sekikawa K (2016) Expression and purification of recombinant mouse interleukin-4 and -6 from transgenic rice seeds. Mol Biotechnol 58:223–231
Goto F, Yoshihara T, Sugimoto N, Toki S, Takaiwa F (1999) Iron fortification of rice seed by the soybean ferritin gene. Nat Biotechnol 17:282–286
Hofbauer A, Stoger E (2013) Subellular accumulation and modification of pharmaceutical proteins in different plant tissues. Curr Pharm Des 19:5495–5502
Kawakatsu T, Wakasa Y, Yasuda H, Takaiwa F (2012) RNA silencing induced by an artificial sequence that prevents proper transcription termination in rice. Plant Physiol 160:601–612
Khan I, Twyman RM, Arcalis E, Stoger E (2012) Using storage organelles for the accumulation and encapsulation of recombinant proteins. Biotechnol J 7:1099–1108
Kingsley DM (1994) The TGF-β superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev 8:133–146
Lau OS, Sun SSM (2009) Plant seeds as bioreactors for recombinant protein production. Biotechnol Adv 27:1015–1022
Mucida D, Park Y, Cheroutre H (2009) From the diet to the nucleus: vitamin A and TGF-β join efforts at the mucosal interface of the intestine. Semin Immunol 21:14–21
Nochi T, Takagi H, Yuki Y, Yang L, Masumura T, Mejima M, Nakanishi U, Matsumura A, Uozumi A, Hiroi T, Morita S, Tanaka K, Takaiwa F, Kiyono H (2007) Rice-based mucosal vaccine as a global strategy for cold-chain- and needle-free vaccination. Proc Natl Acad Sci USA 104:10986–10991
Onda Y, Nagamine A, Sakurai M, Kumamaru T, Ogawa M, Kawagoe Y (2011) Distinct roles of protein disulfide isomerase and P5 sulfhydryl oxidoreductases in multiple pathways for oxidation of structurally diverse storage proteins in rice. Plant Cell 23:210–223
Oono Y, Wakasa Y, Hirose S, Yang L, Sakuta C, Takaiwa F (2010) Analysis of ER stress in developing rice endosperm accumulating β-amyloid peptide. Plant Biotechnol J 8:691–718
Seigneurin-Berny D, Rolland N, Garin J, Joyard J (1999) Differential extraction of hydrophobic proteins from chloroplast envelope membranes: a subcellular-specific proteomic approach to identify rare intrinsic membrane proteins. Plant J 19:217–228
Sharma AK, Sharma MK (2009) Plants as bioreactors: recent developments and emerging opportunities. Biotechnol Adv 27:811–832
Sirko A, Vanek T, Gora-Sochacka A, Redkiewicz P (2011) Recombinant cytokines from plants. Int J Mol Sci 12:3536–3552
Stoger E, Ma JK, Fischer R, Christou P (2005) Sowing the seeds of success: pharmaceutical proteins from plants. Curr Opin Biotechnol 16(2):167–173
Streatfield SJ (2007) Appraches to achieve high-level heterologous protein production in plants. Plant Biotechnol J 5:2–15
Sugita K, Endo-Kasahara S, Tada Y, Lijun Y, Yasuda H, Hayashi Y, Jomori T, Ebinuma H, Takaiwa F (2005) Genetically modified rice seeds accumulating GLP-1 analogue stimulate insulin secretion from a mouse pancreatic beta-cell line. FEBS Lett 579:1085–1088
Tada Y, Usumi S, Takaiwa F (2003) Foreign gene products can be enhanced by introduction into storage protein mutants. Plant Biotechnol J 1:411–422
Takagi H, Saito S, Yang L, Nagasaka S, Nishizawa N, Takaiwa F (2005) Oral immunotherapy against a pollen allergy using a seed-based peptide vaccine. Plant Biotechnol J 3:521–533
Takagi H, Hiroi T, Hirose S, Yang L, Takaiwa F (2010) Rice seed ER-derived protein body as an efficient delivery vehicle for oral tolerogenic peptides. Peptides 31:1421–1425
Takagi H, Watanabe N, Hiroi T, Takaiwa F (2015) Efficacy of transgenic rice containing human interleukin-10 in experimental mouse models of colitis and pollen allergy. Plant Biotechnol 32(4):329–332
Takaiwa F (2013a) Update on the use of transgenic rice seeds in oral immunotherapy. Immunotherapy 5:301–312
Takaiwa F (2013b) Increasing the production yield of recombinant protein in transgenic seeds by expanding the deposition space within the intracellular compartment. Bioengineered 4:136–139
Takaiwa F, Yang L, Yasuda H (2008) Health-promoting transgenic rice seeds as a direct delivery system for bioactive peptides in human health. In: Hirano HY, Hirai A, Sano Y, Sasaki T (eds) Rice biology in the genomics era. Springer, Berlin, pp 357–373
Takaiwa F, Hirose S, Takagi H, Yang L, Wakasa Y (2009) Deposition of a recombinant peptide in ER-derived protein bodies by retention with cysteine-rich prolamins in transgenic rice seed. Planta 229:1147–1158
Wakasa Y, Takaiwa F (2013) The use of rice seeds to produce human pharmaceuticals for oral therapy. Biotechnol J 8:1133–1143
Wakasa Y, Yasuda H, Takaiwa F (2006) High accumulation of bioactive peptide in transgenic rice seeds by expression of introduced multiple genes. Plant Biotechnol J 4:499–510
Wakasa Y, Yasuda H, Oono Y, Kawakatsu T, Hirose S, Takahashi H, Hayashi S, Yang L, Takaiwa F (2011) Expression of ER quality control related genes in response to changes in BiP1 levels in developing rice endosperm. Plant J 65:675–689
Wang S, Takahashi H, Kajiura H, Kawakatsu T, Fujiyama K, Takaiwa F (2013) Transgenic rice seeds accumulating recombinant hypoallergenic birch pollen allergen Bet v 1 generate giant protein bodies. Plant Cell Physiol 54:917–933
Yang L, Suzuki K, Hirose S, Wakasa Y, Takaiwa F (2007) Development of transgenic rice seed accumulating a major Japanese cedar pollen allergen (Cry j 1) structurally disrupted for oral immunotherapy. Plant Biotechnol J 5:815–826
Yang L, Hirose S, Takahashi H, Kawakatsu T, Takaiwa F (2012) Recombinant protein yield in rice seed is enhanced by specific suppression of endogenous seed proteins at the same deposit site. Plant Biotechnol J 10:1035–1045
Yasuda H, Hirose S, Kawakatsu T, Wakasa Y, Takiwa F (2009) Overexpression of BiP has inhibitory effects on the accumulation of seed storage proteins in endosperm cells of rice. Plant Cell Physiol 50:1532–1543
Yoshimura A, Wakabayashi Y, Mori T (2010) Cellular and molecular basis for the regulation of inflammation by TGF-beta. J Biochem 147:781–792
Acknowledgments
We thank Ms. M. Utsuno, Y. Ikemoto, K. Miyashita, and Y. Yajima for technical assistance. This work was supported by ‘Genomics and Agricultural Innovation, GMC0004’ to F.T. from the Ministry of Agriculture Forestry and Fisheries of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by S. Schillberg.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Takaiwa, F., Yang, L., Maruyama, N. et al. Deposition mode of transforming growth factor-β expressed in transgenic rice seed. Plant Cell Rep 35, 2461–2473 (2016). https://doi.org/10.1007/s00299-016-2047-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-016-2047-2