Abstract
Recent rapid progress in plant science and biotechnology in China demonstrates that China’s stronger support for funding in plant research and development (R&D) has borne fruit. Chinese groups have contributed major advances in a range of fields, such as rice biology, plant hormone and developmental biology, genomics and evolution, plant genetics and epigenetics, as well as plant biotechnology. Strigolactone studies including those identifying its receptor and dissecting its complex structure and signaling are representative of the recent researches from China at the forefront of the field. These advances are attributable in large part to interdisciplinary studies among scientists from plant science, chemistry, bioinformatics, structural biology, and agronomy. The platforms provided by national facilities facilitate this collaboration. As well, efficient restructuring of the top–down organization of state programs and free exploration of scientists’ interests have accelerated achievements by Chinese researchers. Here, we provide a general outline of China’s progress in plant R&D to highlight fields in which Chinese research has made significant contributions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
China’s research environment promotes rapid growth in plant R&D
Enough food supply is always one of the most important issues for the government in China because of its huge population. On the other hand, dwindling areas of arable lands and global climate change have challenged amount of the agriculture production. Therefore, Chinese government always has strongly supported the agriculture sciences, as well as plant sciences during the state development.
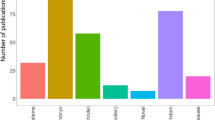
Plant molecular biology has come into a new phase in China (Chen et al. 2006), compared with the last wave, which involved plant tissue culture and its application on main crops, economically important plants, ornamental plants and tree species in the 1980–1990s. Plant research groups at institutes and universities are distributed mainly in five key areas of China: Beijing, Shanghai, Wuhan, Guangdong province and China’s western region, such as Yunnan province due to its rich biodiversity and plant resources. This distribution pattern corresponds to the one described by Prof. Tang Peisong in 1983 (Tang 1983), but there is now a larger population of scientists compared with that in 1980s. As a marker of the progress that has been made, original scientific research articles from Chinese laboratories published in international peer-reviewed journals showed a great change not only in total number but also in quality. In the past 10 years, publications in mainstream journals (those with an impact factor higher than five) from mainland Chinese groups increased more than 4.6-fold (Fig. 1). In particular, papers in the top journals such as Nature, Science, Cell and their sister journals significantly increased. Furthermore, there were more than 40 research articles from Chinese groups in The Plant Cell in 2012. The National Conference on Plant Biology, held annually, is jointly organized by the Chinese Society of Plant Biology, the Botanical Society of China, the Chinese Society of Genetics, the Chinese Society of Cell Biology, and the Chinese Society of Crop Sciences.
Annual plant science publications from China. Annual number of plant science publications originating from China between 2004 and 2012, based on a sample of representative journals that included Nature, Science, Cell and their sister journals, PNAS, EMBO J, The Plant Cell, Plant Physiology, The Plant Journal, PLoS Biology, PLoS Genetics and others with high impact (ISI impact factor greater than 5)
Rice biology is a good example illustrating the speedy progress of research taking place in China. In addition to rice genome sequencing and genome-wide association studies, more than 140 agronomical important genes involved in growth and development, pest resistance and stress tolerance, especially for high-yield traits, were functionally identified (Zuo and Li 2013). More than 2/3 key genes which involved in rice grain yield and published in the top journals have been identified by Chinese groups. Another example is plant reproductive biology, for which the increase of publication numbers from Chinese groups was clearly faster than for plant biology as a whole (Fig. 2) (data from Prof Hong Ma of Fudan University). Percentage of the publication number from Chinese groups in the global total grew up to about 20 % from 7 % in the past decade. In plant biotechnology area, besides plant tissue culture, great achievements have been made in transgenic plants.
China’s stable and high-speed economic development has supported greater funding for scientific research. The total funding for R&D reached up to 2.0 % of the Gross Domestic Product (GDP) in 2012, although percentage of funding for basic research was still lower. Plant science and its related fields have been areas of concern for the government because China perennially faces the problem of ensuring sufficient food supply to its huge population. As a Chinese economic development initiative, funding for plant R&D has been increased rapidly in the last decade (Fig. 3). The funding comes mainly from the National Natural Science Foundation of China (NSFC), the state major basic research program (973 program)and the national high-tech R&D program (863 Program) supported by the Ministry of Science and Technology, with additional R&D funding from the Chinese Academy of Sciences (CAS) and the related ministries. Notably, funding from the NSFC, which supports only basic research, has grown fivefold in the past 5 years. The NSFC has also launched a series of special major research plans, including those focusing on plant hormones (2008–2017) and genetic networks of complex traits in crops (2013–2020). Each plan has a budget of 150–200 million Chinese yuan (about 24–32 million USD). Besides, the national major R&D program for transgenic plants and animals [Phase I (1999–2005); Phase II (2007–2020)] will have total budget of 24 billion Chinese yuan (3.9 billion USD), and has promoted broad scientific research and industrialization. It includes five main crops: cotton, rice, maize, wheat, and soybean. Recently, the CAS launched a strategic priority research program (2013–2018) with designer breeding by molecular modules mainly focusing on rice and wheat. It is clear that the plant species used in the experiments have been gradually migrated from Arabidopsis to rice and other main crops, according to the national food supply needs. Overall, increasing funding from the Chinese government will continue to promote plant science and keep a balance between subject area frontiers and national needs in China.
Publications in plant reproductive and development biology from China (From Dr. Hong Ma, Fudan University). Annual number of plant science publications originating from China from 2004 to 2013, based on a sample of the international journals. The dashed or hollow frame represents supposed value based on the development trends
Genomic and genetic studies are bellwethers of rapid growth in plant science in China
Technical developments in DNA sequencing, proteomics and bioinformatics, as well as plant genetic resources including wild rice and cultivars, and T-DNA insertion lines have shed light on a range of research fields in China (Guo et al. 2006; Wan et al. 2009). Following rice genome sequencing work (Feng et al. 2002; Goff et al. 2002), Chinese groups have published a series of plant genome sequences, such as cucumber (Huang et al. 2009), Chinese cabbage (Wang et al. 2011), potato (Xu et al. 2011), Setaria italica (Zhang et al. 2012), Gossypium raimondii (Wang et al. 2012a), watermelon (Guo et al. 2012), Citrus sinensis (Xu et al. 2012b), Pyrus bretschneideri Rehd. (Wu et al. 2013), salt-tolerant plant Thellungiella salsuginea (Wu et al. 2012), kiwifruit (Actinidia chinensis) (Huang et al. 2013), etc. (some of them collaborated with foreign laboratories). Chinese scientists involved in the tomato genome consortium also participated the sequencing tomato genome (Zouine et al. 2012). Genomic and evolutionary analyses of rice, wheat, potato, cucumber, and plum blossom have been carried out (Jia et al. 2013; Jiao et al. 2012; Ling et al. 2013; Qi et al. 2013; Xiang et al. 2010). Based on genome sequences from 446 geographically diverse accessions of the wild rice species, a rice genome variation map revealed the origin and evolution of cultivated rice (Huang et al. 2012). Cucumber genomic variation map suggests one of the 112 putative domestication sweeps in these regions which contains a gene involved in the loss of bitterness in fruits, an essential domestication trait of cucumber (Qi et al. 2013). Particularly notable accomplishments include the publishing in Nature of draft genomes of wheat A (Triticum urartu) and D (Aegilops tauschii) (Jia et al. 2013; Ling et al. 2013). These draft genome sequences offer diploid references for polyploid wheat genomes, as well as providing insight into the environmental adaptation of bread wheat, and can aid in understanding the large and complicated genomes of wheat species. This progress in genome biology has been enabled by improvements in capacity for sequencing and computational developments, as well as administration and cooperation between companies and institutions (such as CAS, CAAS, universities, and BGI).
High-throughput approaches such as proteomics and transcriptomics have promoted the exploration of fundamental plant biological processes. In the early 2000s, high-throughput technical platforms based on mass spectra were established in institutions such as the Institute of Botany, the Beijing Institute of Genomics, CAS, the Beijing genomics institute (BGI), etc. Absolute quantitation of isoforms of post-translationally-modified proteins has been performed in transgenic organisms (Li et al. 2012). In the past 10 years, 160 groups from 76 institutions in China have published 280 papers on 84 species, including Arabidopsis, rice, wheat, cotton, soybean, maize, and poplar, in this research area. In particular, mechanisms of polar cell growth of pollen tubes, cotton lint and root hairs have been explored by proteomics approaches (Dai et al. 2007; Pang et al. 2010; Xu et al. 2008). Currently, proteomics research teams are working to detail networks of protein interactions including network modifications during growth and development and responses to the environment.
The state major basic research programs (973) for crops include: rice functional genomics and designer breeding by molecular modules, molecular biology of photosynthesis and biological nitrogen fixation, molecular mechanism of plant reproduction and their hormonal regulation, molecular basis of high-efficiency utilization of N and P by crops, molecular basis of pest resistance and stress tolerance, functional genomics of cotton fiber development and quality improvement, molecular biology of wood formation and tree breeding (Populus, Larix), plant secondary metabolism and molecular basis of quality improvement in main vegetables and fruits, molecular regulation of fatty acid biosynthesis in rapeseed, molecular improvement of Cassava, genetics of crop germplasms, etc.
The projects mainly focus on main cereals, such as rice, wheat, maize, and important vegetables, fruit species and woody plants. The progress has been accelerated by functional genomics of crops. For example, functional genomics projects in rice have produced a series of breakthroughs. Yield and fertility are key traits of interest for both rice production and basic research. Of rice yield studies involving seed size, seed number and tillers, the publication from Chinese groups represent about 64 % of the total published in the top journals (impact factor higher than 10). They reported that rice architecture traits (such as tillering and panicle type) affecting yield are controlled by key components such as MOC1/LS/LAS, TAD1, and TE that form a complex involved in APC/C in the cell cycle (Lin et al. 2012; Xu et al. 2012a). In addition, Li’s group demonstrated that a point mutation in OsSPL14 perturbs OsmiR156-directed regulation of OsSPL14, generating an ‘ideal’ rice plant with reduced tiller number, increased lodging resistance and enhanced grain yield. Further, Huang et al. (2009) showed that a DEP1 dominant allele causes shorter inflorescence internodes, more grains per panicle, and a consequent increase in grain yield. Chinese groups also found that artificial selection of an amino acid substitution in PROG1 during domestication led to the transition from the wild rice plant architecture, and affected erect growth and grain yields in cultivated rice (Jin et al. 2008; Tan et al. 2008). It was also reported that the rice GIF1 gene encoding a cell wall invertase is required for carbon partitioning during early grain filling, which is of potential use in breeding (Wang et al. 2008).
Some genetic materials have been used in traditional breeding and rice production in China, despite lack of knowledge of the underlying molecular mechanisms. In recent years, a number of such mysteries have been clarified by Chinese researchers. Chinese super hybrid rice depending on male-sterile lines has been widely used in rice production not only in China but also in other countries for decades. Molecular studies revealed that rice cytoplasmic male sterility (CMS)-related cytoplasmic–nuclear incompatibility is driven by a detrimental interaction between a newly evolved mitochondrial gene (WA352) and a conserved, essential nuclear gene (Cox11) (Luo et al. 2013; Zuo and Li 2013). Another example is photoperiod-sensitive male sterility (PSMS), which has also been used in rice breeding since the 1980s (Ding et al. 2012). As well, a killer–protector system at the S5 locus encoded by three tightly linked genes regulates fertility in indica-japonica hybrids, the information may aid in rice genetic improvement (Yang et al. 2012). The parallel-sequential divergence evolutionary genetic model in the hybrid sterility in rice involves three tightly linked loci, exemplified by a killer–protector system formed of mutations in two steps (Ouyang et al. 2011). The CSA-based photoperiod-sensitive male-sterile line allows the establishment of a stable two-line hybrid system, which promises to have a significant impact on breeding (Zhang et al. 2013).
Rapid progress has also been made in plant epigenetic research in China. Outstanding achievements have come mainly in studies of histone modification, DNA methylation and microRNAs. Specifically, protein arginine methyltransferase, SKB1/PRMT5 and its family members such as PRMT10, were found to mediate histone modification H4R3 and pre-mRNA splicing to control flowering and salt stress responses, as well as stomatal closure in response to Ca2+ in Arabidopsis (Deng et al. 2010; Fu et al. 2013; Wang et al. 2007). In addition, the histone methyltransferase SDG724 targets H3K36me2/3 at MADS50 and RFT1 to promote rice flowering (Sun et al. 2012). Histone demethylases that act on H3K27, such as REF6 in Arabidopsis, and on H3K4 in rice were reported to be involved in the development (Cui et al. 2013; Lu et al. 2011). Epigenome replication was found to be closely linked with DNA replication during S phase (Liu and Gong 2011), and it was revealed that the diRNAs may function as guide molecules directing chromatin modifications or the recruitment of protein complexes to DNA double-strand break sites to facilitate repair (Wei et al. 2012), representing an example of microRNAs mediating plant responses to the environment. Heat stress-induced alternative splicing was found to provide a novel mechanism for regulation of microRNA processing in Arabidopsis (Yan et al. 2012). These important works exemplify the great progress in the plant epigenetic research brought about by young Chinese groups.
There has also been a burst of high-quality Chinese papers on Arabidopsis developmental responses to environmental factors such as light, salt, and drought. Most of them have come from young scientists trained in overseas labs. For example, COP complexes were found to mediate photo- or skoto-morphogenesis. Genome-wide analysis identified transcription regulation networks involving far-red mediated hypocotyl growth (Jing et al. 2013; Ouyang et al. 2011; Tang et al. 2012). Identification of several components involved in plastid retrograde signal generation, transmission, and control of nuclear gene expression has provided significant insight into the regulatory network of plastid retrograde signaling (Chi et al. 2013; Sun et al. 2011). In terms of stress network signaling, a SOS complex and Na+/H+ antiport, as well as DNA methylation and DNA replication, were found to be involved in regulation of salt and drought responses (Gong and Zhu 2011; Ye et al. 2013; Zhou et al. 2012). It is also worth mentioning the findings that kinase CIPK23-mediated complexes and a WRKY transcriptional network (WRKY6, WRKY4, PHO1) function in nutrient stress (Chen et al. 2009; Xu et al. 2006). Phosphoinositide signaling pathway regulates multiple processes of plant growth and development, and cell responses to environmental stimuli in plants (Chen et al. 2008; Xue et al. 2009). For example, phosphatidylinositol pathway-controlled Ins(1,4,5)P(3)/Ca2+ levels are crucial for maintaining pollen dormancy in Arabidopsis (Wang et al. 2012a, b). Crosstalk between the phosphatidylinositol signaling pathway and auxin response is controlled by polar auxin transport (Mei et al. 2011).
In terms of photosynthesis research, China has given rise to a wave of molecular genetics, structural and computational biology studies. Representative of this is the solving of the crystal structure of a spinach major light-harvesting complex at 2.72 A resolution, revealing the first X-ray structure of LHC-II in icosahedral proteoliposome assembly at atomic detail as well as structural insights into energy regulation of LHC-II CP29 (Liu et al. 2004; Pan et al. 2011). In addition, LTD (light-harvesting chlorophyll-binding protein translocation defect) was reported to be essential for the import of light-harvesting chlorophyll-binding proteins and subsequent routing of these proteins to the chloroplast signal recognition particle-dependent pathway (Ouyang et al. 2011). Identification of several components in the plastid retrograde generation, transmission, and control of nuclear genes expression has provided significant insight into the regulatory network of plastid retrograde signaling in Arabidopsis (Chi et al. 2013).
Within plant development biology, important advances include the recognition mechanism during pollination and the genetic network underlying organogenesis (Zhang et al. 2009). Their results have provided insight into S-RNase-based self-incompatibility in flowering plants via SLF-mediated degradation (Chen and Qiu 2012; Zhang et al. 2009), the involvement of small peptides in gametophyte and pollination recognition (Liu et al. 2013; Yang et al. 2010), as well as floral organogenesis. A model of S-RNase-based self-incompatibility involving SLF-mediated degradation, based on the studies on snapdragon, was proposed to explain the biochemical mechanism for specific rejection of self-pollen tubes by the pistil (Xu et al. 2013). Pollination and cell cytoskeleton research are examples reflecting the development of cell biological research to include not only classical functional research into the cytoskeleton but also examination of novel functions of transcription factors in cellular processes involving hormone biosynthesis and signaling, such as of GAs and BRs (Li et al. 2011a; Wang et al. 2012b). In cucumber unisexual flower development, it is interesting to demonstrate at molecular level that ethylene selectively promotes female flower formation by inhibiting stamen development (Sun et al. 2010).
Although genetic and molecular networks on vernalization have been identified in Arabidopsis, cereals as well as biennial-to-perennial plants are of diverse patterns to control vernalization-required flowering. Chinese groups have shown a significant progress. An example is molecular mechanism studies on vernalization for flowering in winter wheat, which is unique, totally different from that in Arabidopsis, not only in the kinds of genes but also in network of the genes. Wheat lectin gene VER2 could accelerate vernalization-mediated flowering (Yong et al. 2003). Lectin VER2 protein recognizing O-GlcNAc signaling on the key protein complex is involved in wheat vernalization. The findings open the way to studies of O-GlcNAc protein modification in response to environmental signals in plant development, which pattern may be shared in organisms (Lee and Shin 2009; Xing et al. 2009). Another example is that the floral transition of Cardamine flexuosa, a herbaceous biennial-to-perennial plant, requires vernalization. The levels of two age-regulated microRNAs, miR156 and miR172, regulate the timing of sensitivity in response to vernalization. Age and vernalization pathways coordinately regulate flowering through modulating the expression of CfSOC1, a flowering-promoting MADS-box gene. The related annual Arabidopsis thaliana, which has both vernalization and age pathways, does not possess an age-dependent vernalization response. Thus, the recruitment of age cue in response to environmental signals contributes to the evolution of life cycle in plants (Zhou et al. 2013a).
Plant hormone researches from Chinese groups highlight the advantages
Hormone researches in China have been promoted by national research programs like the major special program on hormone function and mechanism from the NSFC and some 973 projects. These programs have attracted scientists from different fields (such as computation science, chemistry and structural biology, as well as agronomy) to solve frontier issues in hormone biology. Various hormone receptors such as COI1 for jasmonic acids (Jas), D14 complex for strigolactone (SL), and PYR/PYL/RCAR and ABAR for abscisic acid have been identified (Shang et al. 2010; Shen et al. 2006; Yan et al. 2009; Zhao et al. 2013). In addition, the newly identified hormone SL was found to function in differentiation of axillary buds in plants (Lin et al. 2009). Recently, two Nature papers from Chinese groups revealed that D14-SCFD3-dependent degradation of D53, as a repressor of SL signaling, regulates rice differentiation of axillary buds for tillering (Jiang et al. 2013; Zhou et al. 2013a, b). At least three aspects of China’s hormone research should be highlighted: the development of an analysis system for trace identification of hormones, the study of metabolism and signaling in plant development, and structure biological approaches revealing molecular mechanisms that underlie hormone functions.
Quantitative chemical identification of trace amounts of complex hormones has been a bottleneck hindering hormone research in past years. Now, Chinese chemists have joined the hormone research field to set up a mass spectrum (MS) system for analysis of complex hormones such as gibberellic acids (GAs) and SLs, and to create novel identification methods. For example, Feng’s group (Wuhan University) has established a MS-based method for quantitative identification of multiple GAs (up to 11 derivatives of GAs) in one sample (Li et al. 2012). In addition, a novel amperometric immunosensor for the phytohormone ABA was created based on chemical reductive growth in situ of gold nanoparticles on glassy carbon electrodes (Wang et al. 2009). Chinese labs have also produced great advances in hormone metabolism and modification, signal transduction, and hormone crosstalk, producing more than 300 publications including original papers and invited reviews in key international journals.
Structural biologists in China have collaborated with plant biologists to discover those key protein complexes in hormone signaling pathways. For instance, the structure of the ABA receptor, the PYR/PYL/RCAR complex, was resolved to clarify the mechanism of coordination between ABA and the complex (Hao et al. 2011). Structural analysis also provided insight into brassinolide perception, revealing that BAK1 is a co-receptor that recognizes the BRI1-bound brassinolide (She et al. 2011; Sun et al. 2013). In addition, the crystal structure of the two hormones signal-transducing α/β hydrolases, karrikin-signaling Kai2 and SL receptor D14 was resolved by the Xu and Li groups (Zhao et al. 2013).
From tissue culture to transgenic crops
Research progress in plant tissue culture has been based on achievements of hormone studies from the 1970s in China. Until the 1990s, China was one of the leading countries in plant tissue culture and its applications. The tissue culture system has been approached to molecular mechanism studies on development, such as apical meristem differentiation using Arabidopsis plantlets. Using the tissue culture system, for example, it has been identified that the establishment of auxin gradients and PIN1-mediated polar auxin transport is essential for WUS induction and somatic embryogenesis in Arabidopsis (Su et al. 2009). As well, DNA methylation and histone modifications regulate de novo shoot regeneration by modulating WUS expression and auxin signaling (Li et al. 2011b). In recent decades, on the other hand, plant tissue culture techniques have been extensively used for a range of important crops and economic plants (Xu 2007). An example is micropropagation for Eucalypyus in Guanxi, plantlets from micropropagation tissue culture of 100 elite individual trees in the past 30 years, and annual plantlets production reaches 200 million in culture jars and 160 million provided for planting in field (Wang et al. 2007). The 2-year seed potato system ensures reduction of virus infection and hence the improvement of seed tuber quality. Virus-tested minituber of potato was applied in about 20 % potato production field in China (Liu et al. 2006). The micropropagation technique has been also used for banana, strawberry, various species and varieties of orchids in horticulture, Dendrobium officinale (Chinese medicinal orchid, Wu Ping, personal communication) and Dioscorea opposite (Li 2004), etc. Anther tissue culture has been successfully used to obtain dihaploid plants for breeding work of maize, rice, wheat and rapeseed, etc. Somatic hybrids by protoplast fusion have been used to obtain new breeding materials in wheat (Xia Guangmin’s Lab), rapeseed, potato and Citrus (Xu et al. 2012b).
Among genetic-modified crops, transgenic cotton has been planted in China for the resistance to insects as well as improvement of fiber quality. Bt insect-resistant transgenic cotton from Chinese breeders, approved in 1997 for commercial use to control cotton bollworm, has been widely used in production. In China, Bt cotton was steadily adopted by the bulk of growers (i.e., presently 95 % adoption in northern China, and 85.65 % for whole country) (Lu et al. 2010). In addition, transgenic maize harboring the phytase gene (BVLA430101) and transgenic rice with insect resistance (Huahui No. 1; Bt Xianyou 63) have obtained safety certification for production according to state regulations, although these have not been commercially planted in China. Risk assessment analyses of the transgenic crops suggest that natural refuges derived from the mixed-planting system of cotton, corn, soybean, and peanut on small-scale, single family-owned farms have a key function in delaying evolution of cotton bollworm resistance, and no trend toward Bt cotton resistance has been apparent despite intensive planting of Bt cotton in recent years (Lu et al. 2010).
Insect resistance in cotton also has been achieved by silencing a cotton bollworm P450 monooxygenase gene using plant-mediated RNAi, thereby impairing larval tolerance of gossypol (Mao et al. 2007). Another example of the improvement of cotton involves spatiotemporal manipulation of auxin biosynthesis in cotton ovule epidermal cells to enhance fiber yield and quality (Zhang et al. 2011). The lint percentage, an important component of fiber yield, is consistently higher in the transgenic plants than in non-transgenic controls, which leads to a more than 15 % increase in yield. This shows a potential for improving cotton production (Chen et al. 2010).
The Wx (waxy) locus controls amylose synthesis in rice and many other species. Wx gene expression can be regulated by antisence approach or RNAi technique in transgenic rice and cassava, which have been used to regulate starch composition. Rice endosperm has been used as bioreactor to produce HSA (human serum albumin) by transgenic rice with high expression of recombinant HAS (OsrHSA) (Yang Daichang’s lab, Wuhan University). In medicinal plant, Artemisia annua, the content of biological active component, artemisinin, is less 1 % (dry matter), but it reached 2.8 % in some strains of transgenic plants with SQSi, which has been proved for field test (Tang Kexuan’s lab, Shanghai Jiaotong University).
Perspective
Since the new century started, the community of plant scientists has steadily enlarged in China. Correspondingly, the output of Chinese plant science research is impressive. Mainland Chinese plant researchers have published a significantly increasing share of the research articles in top journals, but breakthroughs driven by original innovations that are capable of leading new research fields and great strides in research methods are still lacking. With continuous support from the Chinese government in terms of increased funding allocations and improved infrastructure, we would like to see that Chinese plant scientists may contribute more than ever to the plant science in the world.
Using the model plant Arabidopsis, Chinese plant scientists have produced high-quality, internationally acknowledged work. However, to feed the 1.3 billon people in China and supply more food to the world despite the shrinking area of arable land, always is one of the top concerns of the Chinese government and politicians, and the responsibility of plant scientists in the world. Plant scientists need to pay more attention for staple crops such as rice, wheat, corn, soybean, rapeseed and cotton. Also, we should study those crops, like potato, sweet potato and cassava, which generally are important food resources for developing regions. When we consider to continue to increase the crop yield, we also should consider to breed more crop varieties which are environmental friendly, that means less chemical fertilizers and pesticides used. With improvement of living standard in China, Chinese scientists are to pay more attention to important vegetables and fruits for improvement of nutrient composition. For identification of active component of Chinese medicinal plants, we seriously lack the knowledge about secondary metabolism for those plants. Thus, it is important to study plant development and hormone function, plant metabolism including photosynthesis, pest resistance and stress tolerance at molecular level, and clone more functionally important genes from germplasm collection. Although many Chinese plant scientists have done impressive work in the above research areas using different crops, they should integrate their research resources, they need more interdisciplinary cooperation to solve those important plant biology problems and provide more molecular breeding approaches and to resolve major agricultural challenges. We are sure that better plant science for better life of human being.
References
Chen YS, Qiu XB (2012) Transcription-coupled replacement of histones: degradation or recycling? JGG 39:575–580
Chen HD, Karplus VJ, Ma H, Deng XW (2006) Plant biology research comes of age in China. Plant Cell 18:2855–2864
Chen X, Lin WH, Wang Y, Luan S, Xue HW (2008) An inositol polyphosphate 5-phosphatase functions in PHOTOTROPIN1 signaling in Arabidopis by altering cytosolic Ca2+. Plant Cell 20:353–366
Chen YF, Li LQ, Xu Q, Kong YH, Wang H, Wu WH (2009) The WRKY6 transcription factor modulates PHOSPHATE1 expression in response to low Pi stress in Arabidopsis. Plant Cell 21:3554–3566
Chen XY, Mao YB, Lin ZP, Wang LJ (2010) Method for modifying insect resistance of plants by utilizing RNAi technique. JUSIA Pat 2010(0050):2294
Chi W, Sun XW, Zhang LX (2013) Intracellular signaling from plastid to nucleus. Annu Rev Plant Biol 64:559–582
Cui XK, Jin P, Cui X, Gu LF, Lu ZK, Xue YM, Wei LY, Qi JF, Song XW, Luo M (2013) Control of transposon activity by a histone H3K4 demethylase in rice. Proc Natl Acad Sci USA 110:1953–1958
Dai SJ, Chen TT, Chong K, Xue YB, Liu SQ, Wang T (2007) Proteomics identification of differentially expressed proteins associated with pollen germination and tube growth reveals characteristics of germinated Oryza sativa pollen. Mol Cell Proteomics 6:207–230
Deng X, Gu LF, Liu CY, Lu TC, Lu FL, Lu ZK, Cui P, Pei YX, Wang BC, Hu SN (2010) Arginine methylation mediated by the Arabidopsis homolog of PRMT5 is essential for proper pre-mRNA splicing. Proc Natl Acad Sci USA 107:19114–19119
Ding JH, Lu Q, Ouyang YD, Mao HL, Zhang PB, Yao JL, Xu CG, Li XH, Xiao JH, Zhang QF (2012) A long noncoding RNA regulates photoperiod-sensitive male sterility, an essential component of hybrid rice. Proc Natl Acad Sci USA 109:2654–2659
Feng Q, Zhang YJ, Hao P, Wang SY, Fu G, Huang YC, Li Y, Zhu JJ, Liu YL, Hu X (2002) Sequence and analysis of rice chromosome 4. Nature 420:316–320
Fu YL, Zhang GB, Lv XF, Guan Y, Yi HY, Gong JM (2013) Arabidopsis histone methylase CAU1/PRMT5/SKB1 acts as an epigenetic suppressor of the calcium signaling gene CAS to mediate stomatal closure in response to extracellular calcium. Plant Cell 25:2878–2891
Goff SA, Ricke D, Lan TH, Presting G, Wang R, Dunn M, Glazebrook J, Sessions A, Oeller P, Varma H (2002) A draft sequence of the rice genome (Oryza sativa L. ssp. japonica). Science 296:92–100
Gong ZZ, Zhu JK (2011) Active DNA demethylation by oxidation and repair. Cell Res 21:1649–1651
Guo LB, Chu CC, Qian Q (2006) Rice mutants and functional genomics. Chin Bulletin Bot 23:1–13
Guo SG, Zhang JG, Sun HH, Salse J, Lucas WJ, Zhang HY, Zheng Y, Mao LY, Ren Y, Wang ZW (2012) The draft genome of watermelon (Citrullus lanatus) and resequencing of 20 diverse accessions. Nat Genet 45:51–58
Hao Q, Yin P, Li WQ, Wang L, Yan CY, Lin ZH, Wu JZ, Wang JW, Yan SF, Yan N (2011) The molecular basis of ABA-independent inhibition of PP2Cs by a subclass of PYL proteins. Mol Cell 42:662–672
Huang SW, Li RQ, Zhang ZH, Li L, Gu XF, Fan W, Lucas WJ, Wang XW, Xie BY, Ni PX (2009) The genome of the cucumber, Cucumis sativus L. Nat Genet 41:1275–1281
Huang XH, Kurata N, Wei XH, Wang ZX, Wang AH, Zhao Q, Zhao Y, Liu KY, Lu HY, Li WJ (2012) A map of rice genome variation reveals the origin of cultivated rice. Nature 490:497–501
Huang S, Ding J, Deng D, Tang W, Sun H, Liu D, Zhang L, Niu X, Zhang X, Meng M, Yu J, Liu J, Han Y, Shi W, Zhang D, Cao S, Wei Z, Cui Y, Xia Y, Zeng H, Bao K, Lin L, Min Y, Zhang H, Miao M, Tang X, Zhu Y, Sui Y, Li G, Sun H, Yue J, Sun J, Liu F, Zhou L, Lei L, Zheng X, Liu M, Huang L, Song J, Xu C, Li J, Ye K, Zhong S, Lu BR, He G, Xiao F, Wang HL, Zheng H, Fei Z, Liu Y (2013) Draft genome of the kiwifruit Actinidia chinensis. Nat Commun 4:2640. doi:10.1038/ncomms3640
Jia JZ, Zhao SC, Kong XY, Li YR, Zhao GY, He WM, Appels R, Pfeifer M, Tao Y, Zhang XY (2013) Aegilops tauschii draft genome sequence reveals a gene repertoire for wheat adaptation. Nature 496:91–95
Jiang L, Liu X, Xiong GS, Liu HH, Chen FL, Wang L, Meng XB, Liu GF, Yu H, Yuan YD (2013) DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature doi:10.1038/nature12870
Jiao YP, Zhao HN, Ren LH, Song WB, Zeng B, Guo JJ, Wang BB, Liu ZP, Chen J, Li W (2012) Genome-wide genetic changes during modern breeding of maize. Nat Genet 44:812–815
Jin J, Huang W, Gao JP, Yang J, Shi M, Zhu MZ, Luo D, Lin HX (2008) Genetic control of rice plant architecture under domestication. Nat Genet 40:1365–1369
Jing YJ, Zhang D, Wang X, Tang WJ, Wang WQ, Huai JL, Xu G, Chen DQ, Li YL, Lin RC (2013) Arabidopsis chromatin remodeling factor PICKLE interacts with transcription factor HY5 to regulate hypocotyl cell elongation. Plant Cell 25:242–256
Lee I, Shin J (2009) F1000Prime Recommendation of [Xing L et al., PLoS One 2009, 4(3):e4854]. In F1000 Prime. doi:10.3410/f.1163821.1625627.F1161000Prime.com/1163821#eval1625627
Li MJ (2004) Dioscorea opposite: tissue culture and its application. Science Press, Marrickville, pp 156–160
Li J, Jiang JF, Qian Q, Xu YY, Zhang C, Xiao J, Du C, Luo W, Zou GX, Chen ML (2011a) Mutation of rice BC12/GDD1, which encodes a kinesin-like protein that binds to a GA biosynthesis gene promoter, leads to dwarfism with impaired cell elongation. Plant Cell 23:628–640
Li W, Liu H, Cheng ZJ, Su YH, Han HN, Zhang Y, Zhang XS (2011b) DNA methylation and histone modifications regulate de novo shoot regeneration in Arabidopsis by modulating WUSCHEL expression and auxin signaling. PLoS Genet. e1002243. doi:10.1371/journal.pgen.1002243
Li YJ, Shu YW, Peng CC, Zhu L, Guo GY, Li N (2012) Absolute quantitation of isoforms of post-translationally modified proteins in transgenic organism. Mol Cell Proteomics 11:272–285
Lin H, Wang RX, Qian Q, Yan MX, Meng XB, Fu ZM, Yan CY, Jiang B, Su Z, Li JY (2009) DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell 21:1512–1525
Lin QB, Wang D, Dong H, Gu SH, Cheng ZJ, Gong J, Qin RZ, Jiang L, Li G, Wang JL (2012) Rice APC/CTE controls tillering by mediating the degradation of MONOCULM 1. Nat Commun 3:752–757
Ling HQ, Zhao SC, Liu DC, Wang JY, Sun H, Zhang C, Fan HJ, Li D, Dong LL, Tao Y (2013) Draft genome of the wheat A-genome progenitor Triticum urartu. Nature 496:87–90
Liu Q, Gong ZZ (2011) The coupling of epigenome replication with DNA replication. Curr Opin Plant Biol 14:187–194
Liu ZF, Yan HC, Wang KB, Kuang TY, Zhang JP, Gui LL, An XM, Chang WR (2004) Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature 428:287–292
Liu J, Nie BH, Cai XK, Chen L, Xie CH (2006) Establishment of two-year seed potato system and improvement of the key techniques. Chin Potato (in Chinese) 20:321–325
Liu XM, Qin T, Ma QQ, Sun J, Liu ZQ, Yuan M, Mao TL (2013) Light-regulated hypocotyl elongation involves proteasome-dependent degradation of the microtubule regulatory protein WDL3 in Arabidopsis. Plant Cell 25:1740–1755
Lu YH, Wu KM, Jiang YY, Xia B, Li P, Feng HQ, Wyckhuys KA, Guo YY (2010) Mirid bug outbreaks in multiple crops correlated with wide-scale adoption of Bt cotton in China. Science 328:1151–1154
Lu FL, Cui X, Zhang SB, Jenuwein T, Cao XF (2011) Arabidopsis REF6 is a histone H3 lysine 27 demethylase. Nat Genet 43:715–719
Luo DP, Xu H, Liu ZL, Guo JX, Li HY, Chen LT, Fang C, Zhang QY, Bai M, Yao N (2013) A detrimental mitochondrial-nuclear interaction causes cytoplasmic male sterility in rice. Nat Genet 45:573–577
Mao YB, Cai WJ, Wang JW, Hong GJ, Tao XY, Wang LJ, Huang YP, Chen XY (2007) Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat Biotech 25:1307–1313
Mei Y, Jia WJ, Chu YJ, Xue HW (2011) Arabidopsis phosphatidylinositol monophosphate 5-kinase 2 is involved in root gravitropism through regulation of polar auxin transport by affecting the cycling of PIN proteins. Cell Res 22:581–597
Ouyang M, Li XY, Ma JF, Chi W, Xiao JW, Zou MJ, Chen F, Lu CM, Zhang LX (2011) LTD is a protein required for sorting light-harvesting chlorophyll-binding proteins to the chloroplast SRP pathway. Nat Commun 2:277–283
Pan XW, Li M, Wan T, Wang LF, Jia CJ, Hou ZQ, Zhao XL, Zhang JP, Chang WR (2011) Structural insights into energy regulation of light-harvesting complex CP29 from spinach. Nat Struct Mol Biol 18:309–315
Pang CY, Wang H, Pang Y, Xu C, Jiao Y, Qin YM, Western TL, Yu SX, Zhu YX (2010) Comparative proteomics indicates that biosynthesis of pectic precursors is important for cotton fiber and Arabidopsis root hair elongation. Mol Cell Proteomics 9:2019–2033
Qi JJ, Liu X, Shen D, Miao H, Xie BY, Li XX, Zeng P, Wang SH, Shang Y, Gu XF (2013) A genomic variation map provides insights into the genetic basis of cucumber domestication and diversity. Nat Genet 45:1510–1515
Shang Y, Yan L, Liu ZQ, Cao Z, Mei C, Xin Q, Wu FQ, Wang XF, Du SY, Jiang T (2010) The Mg-chelatase H subunit of Arabidopsis antagonizes a group of WRKY transcription repressors to relieve ABA-responsive genes of inhibition. Plant Cell 22:1909–1935
She J, Han ZF, Kim TW, Wang JJ, Cheng W, Chang JB, Shi S, Wang JW, Yang MJ, Wang ZY (2011) Structural insight into brassinosteroid perception by BRI1. Nature 474:472–476
Shen YY, Wang XF, Wu FQ, Du SY, Cao Z, Shang Y, Wang XL, Peng CC, Yu XC, Zhu SY (2006) The Mg-chelatase H subunit is an abscisic acid receptor. Nature 443:823–826
Su YH, Zhao XY, Liu YB, Zhang CL, O’Neill SD, Zhang XS (2009) Auxin-induced WUS expression is essential for embryonic stem cell renewal during somatic embryogenesis in Arabidopsis. Plant J 59(3):448–460. doi:10.1111/j.1365-313X.2009.03880.x
Sun JJ, Li F, Li X, Liu XC, Rao GY, Luo JC, Wang DH, Xu ZH, Bai SN (2010) Why is ethylene involved in selective promotion of female flower development in cucumber? Plant Signal Behav 5:1052–1056
Sun XW, Feng PQ, Xu XM, Guo HL, Ma JF, Chi W, Lin RC, Lu CM, Zhang LX (2011) A chloroplast envelope-bound PHD transcription factor mediates chloroplast signals to the nucleus. Nat Commun 2:477–483
Sun CH, Fang J, Zhao TL, Xu B, Zhang FT, Liu LC, Tang JY, Zhang GF, Deng XJ, Chen F (2012) The histone methyltransferase SDG724 mediates H3K36me2/3 deposition at MADS50 and RFT1 and promotes flowering in rice. Plant Cell 24:3235–3247
Sun YD, Han ZF, Tang J, Hu ZH, Chai CL, Zhou B, Chai JJ (2013) Structure reveals that BAK1 as a co-receptor recognizes the BRI1-bound brassinolide. Cell Res 23:1326–1329
Tan LB, Li XR, Liu FX, Sun XY, Li CG, Zhu ZF, Fu YC, Cai HW, Wang XK, Xie DX (2008) Control of a key transition from prostrate to erect growth in rice domestication. Nat Genet 40:1360–1364
Tang PS (1983) Aspirations, reality, and circumstances: the devious trail of a roaming plant physiologist. Annual Rev Plant Physiol 34:1–20
Tang WJ, Wang WQ, Chen DQ, Ji Q, Jing YJ, Wang HY, Lin RC (2012) Transposase-derived proteins FHY3/FAR1 interact with PHYTOCHROME-INTERACTING FACTOR1 to regulate chlorophyll biosynthesis by modulating HEMB1 during deetiolation in Arabidopsis. Plant Cell 24:1984–2000
Wan SY, Wu JX, Zhang ZG, Sun XH, Lv YC, Gao C, Ning YD, Ma J, Guo YP, Zhang Q (2009) Activation tagging, an efficient tool for functional analysis of the rice genome. Plant Mol Biol 69:69–80
Wang X, Zhang Y, Ma QB, Zhang ZL, Xue YB, Bao SL, Chong K (2007) SKB1-mediated symmetric dimethylation of histone H4R3 controls flowering time in Arabidopsis. EMBO J 26:1934–1941
Wang E, Wang JJ, Zhu XD, Hao W, Wang LY, Li Q, Zhang LX, He W, Lu BR, Lin HX (2008) Control of rice grain-filling and yield by a gene with a potential signature of domestication. Nat Genet 40:1370–1374
Wang RZ, Li YW, Li Q, Shen GL, Xiao LT (2009) A novel amperometric immunosensor for phytohormone abscisic acid based on in situ chemical reductive growth of gold nanoparticles on glassy carbon electrode. Analytical Lett 42:2893–2904
Wang XW, Wang HZ, Wang J, Sun RF, Wu J, Liu SY, Bai YQ, Mun JH, Bancroft I, Cheng F (2011) The genome of the mesopolyploid crop species Brassica rapa. Nat Genet 43:1035–1039
Wang KB, Wang ZW, Li FG, Ye WW, Wang JY, Song GL, Yue Z, Cong L, Shang HH, Zhu SL (2012a) The draft genome of a diploid cotton Gossypium raimondii. Nat Genet 43:1035–1039
Wang Y, Chu YJ, Xue HW (2012b) Inositol polyphosphate 5-phosphatase-controlled Ins(1,4,5)P3/Ca2+ is crucial for maintaining pollen dormancy and regulating early germination of pollen. Development 139:2221–2233
Wei W, Ba ZQ, Gao M, Wu Y, Ma YT, Amiard S, White CI, Rendtlew Danielsen JM, Yang YG, Qi YJ (2012) A role for small RNAs in DNA double-strand break repair. Cell 149:101–112
Wu HJ, Zhang ZH, Wang JY, Oh DH, Dassanayake M, Liu BH, Huang QF, Sun HX, Xia R, Wu YR (2012) Insights into salt tolerance from the genome of Thellungiella salsuginea. Proc Natl Acad Sci USA 109:12219–12224
Wu J, Wang ZW, Shi ZB, Zhang S, Ming R, Zhu SL, Khan MA, Tao ST, Korban SS, Wang H (2013) The genome of the pear (Pyrus bretschneideri Rehd.). Genome Res 23:396–408
Xiang H, Zhu J, Chen Q, Dai F, Li X, Li M, Zhang H, Zhang G, Li D, Dong Y (2010) Single base-resolution methylome of the silkworm reveals a sparse epigenomic map. Nature Biot 28:516–520
Xing LJ, Li J, Xu YY, Xu ZH, Chong K (2009) Phosphorylation modification of wheat lectin VER2 is associated with vernalization-induced O-GlcNAc signaling and intracellular motility. PLoS One 4:e4854
Xu ZH (2007) Plant biotechnology and crop improvement in China. In: Biotechnology and sustainable agriculture 2006 and Beyond. Springer, pp 3–10
Xu J, Li HD, Chen LQ, Wang Y, Liu LL, He L, Wu WH (2006) A protein kinase, interacting with two Calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 125:1347–1360
Xu SB, Li T, Deng ZY, Chong K, Xue YB, Wang T (2008) Dynamic proteomic analysis reveals a switch between central carbon metabolism and alcoholic fermentation in rice filling grains. Plant Physiol 148:908–915
Xu X, Pan SK, Cheng SF, Zhang B, Mu DS, Ni PX, Zhang GY, Yang S, Li RQ, Wang J (2011) Genome sequence and analysis of the tuber crop potato. Nature 475:189–195
Xu C, Wang YH, Yu YC, Duan JB, Liao ZG, Xiong GS, Meng XB, Liu GF, Qian Q, Li JY (2012a) Degradation of MONOCULM 1 by APC/CTAD1 regulates rice tillering. Nat Comm 3:750–758
Xu Q, Chen LL, Ruan XA, Chen DJ, Zhu AD, Chen CL, Bertrand D, Jiao WB, Hao BH, Lyon MP (2012b) The draft genome of sweet orange (Citrus sinensis). Nat Genet 45:59–66
Xu C, Li MF, Wu JK, Guo H, Li Q, Zhang YE, Chai JJ, Li TZ, Xue YB (2013) Identification of a canonical SCF(SLF) complex involved in S-RNase-based self-incompatibility of Pyrus (Rosaceae). Plant Mol Biol 81:245–257
Xue HW, Chen X, Mei Y (2009) Function and regulation of phospholipid signalling in plants. Biochem J 421:145–156
Yan JB, Zhang C, Gu M, Bai ZY, Zhang WG, Qi TC, Cheng ZW, Peng W, Luo HB, Nan FJ (2009) The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell 21:2220–2236
Yan K, Liu P, Wu CA, Yang GD, Xu R, Guo QH, Huang JG, Zheng CC (2012) Stress-induced alternative splicing provides a mechanism for the regulation of MicroRNA processing in Arabidopsis thaliana. Mol Cell 48:521–531
Yang WC, Shi DQ, Chen YH (2010) Female gametophyte development in flowering plants. Annu Rev Plant Biol 61:89–108
Yang JY, Zhao XB, Cheng K, Du HY, Ouyang YD, Chen JJ, Qiu SQ, Huang JY, Jiang YH, Jiang LW (2012) A killer-protector system regulates both hybrid sterility and segregation distortion in rice. Science 337:1336–1340
Ye JM, Zhang WH, Guo Y (2013) Arabidopsis SOS3 plays an important role in salt tolerance by mediating calcium-dependent microfilament reorganization. Plant Cell Rep 32:139–148
Yong WD, Xu YY, Xu WZ, Wang X, Li N, Wu JS, Liang TB, Chong K, Xu ZH, Tan KH, Zhu ZQ (2003) Vernalization-induced flowering in wheat is mediated by a lectin-like gene VER2. Planta 217(2):261–270
Zhang Y, Zhao ZH, Xue YB (2009) Roles of proteolysis in plant self-incompatibility. Annu Rev Plant Biol 60:21–42
Zhang M, Zheng XL, Song SQ, Zeng QW, Hou L, Li DM, Zhao J, Wei Y, Li XB, Luo M (2011) Spatiotemporal manipulation of auxin biosynthesis in cotton ovule epidermal cells enhances fiber yield and quality. Nat Biotechnol 29:453–458
Zhang GY, Liu X, Quan ZW, Cheng SF, Xu X, Pan SK, Xie M, Zeng P, Yue Z, Wang WL (2012) Genome sequence of foxtail millet (Setaria italica) provides insights into grass evolution and biofuel potential. Nat Biotechnol 30:549–554
Zhang H, Xu CX, He Y, Zong J, Yang XJ, Si HM, Sun ZX, Hu JP, Liang WQ, Zhang DB (2013) Mutation in CSA creates a new photoperiod-sensitive genic male sterile line applicable for hybrid rice seed production. Proc Natl Acad Sci USA 110:76–81
Zhao LH, Zhou XE, Wu ZS, Yi W, Xu Y, Li SL, Xu TH, Liu Y, Chen RZ, Kovach A (2013) Crystal structures of two phytohormone signal-transducing α/β hydrolases: karrikin-signaling KAI2 and strigolactone signaling DWARF14. Cell Res 23:436–439
Zhou HP, Zhao JF, Yang YQ, Chen CX, Liu YF, Jin XH, Chen LM, Li XY, Deng XW, Schumaker KS (2012) UBIQUITIN-SPECIFIC PROTEASE16 modulates salt tolerance in Arabidopsis by regulating Na+/H+ antiport activity and serine hydroxymethyltransferase stability. Plant Cell 24:5106–5122
Zhou CM, Zhang TQ, Wang X, Yu S, Lian H, Tang H, Feng ZY, Zozomova-Lihová J, Wang JW (2013a) Molecular basis of age-dependent vernalization in Cardamine flexuosa. Science 340(6136):1097–1100. doi:10.1126/science.1234340
Zhou F, Lin QB, Zhu LH, Ren YL, Zhou KN, Shabek N, Wu FQ, Mao HB, Dong W, Gan L (2013b) D14-SCFD3-dependent degradation of D53 regulates strigolactone signalling. Nature. doi:10.1038/nature12878
Zouine M, Latché A, Rousseau C, Regad F, Pech J-C, Philippot M, Bouzayen M, Delalande C, Frasse P, Schiex T (2012) The tomato genome sequence provides insights into fleshy fruit evolution. Nature 485:635–641
Zuo JR, Li JY (2013) Molecular dissection of complex agronomic traits of rice: a team effort by Chinese scientists in recent years. Natl Sci Rev 00:1–24
Acknowledgments
The authors thank Dr. Hong Ma (Fudan University), Dr. Yongbiao Xue (Institute of Genetics and Development Biology, CAS), Dr. Tai Wang and Dr. Lei Wang (Institute of Botany, CAS) for their information and comments, and a lot of Chinese colleagues for providing their research results. We also thank Dr. Ke Li for her assistance in preparing the manuscript. We thank the Chinese Scientists for their understanding that the commentary may miss some key works since the limited publication space.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by X. S. Zhang.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Chong, K., Xu, Z. Investment in plant research and development bears fruit in China. Plant Cell Rep 33, 541–550 (2014). https://doi.org/10.1007/s00299-014-1587-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-014-1587-6