Abstract
Key message
RNAi-mediated suppression of the endogenous storage proteins in MucoRice-CTB-RNAi seeds affects not only the levels of overexpressed CTB and RAG2 allergen, but also the localization of CTB and RAG2.
Abstract
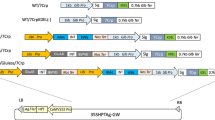
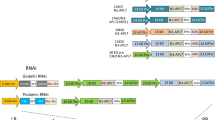
A purification-free rice-based oral cholera vaccine (MucoRice-CTB) was previously developed by our laboratories using a cholera toxin B-subunit (CTB) overexpression system. Recently, an advanced version of MucoRice-CTB was developed (MucoRice-CTB-RNAi) through the use of RNAi to suppress the production of the endogenous storage proteins 13-kDa prolamin and glutelin, so as to increase CTB expression. The level of the α-amylase/trypsin inhibitor-like protein RAG2 (a major rice allergen) was reduced in MucoRice-CTB-RNAi seeds in comparison with wild-type (WT) rice. To investigate whether RNAi-mediated suppression of storage proteins affects the localization of overexpressed CTB and major rice allergens, we generated an RNAi line without CTB (MucoRice-RNAi) and investigated gene expression, and protein production and localization of two storage proteins, CTB, and five major allergens in MucoRice-CTB, MucoRice-CTB-RNAi, MucoRice-RNAi, and WT rice. In all lines, glyoxalase I was detected in the cytoplasm, and 52- and 63-kDa globulin-like proteins were found in the aleurone particles. In WT, RAG2 and 19-kDa globulin were localized mainly in protein bodies II (PB-II) of the endosperm cells. Knockdown of glutelin A led to a partial destruction of PB-II and was accompanied by RAG2 relocation to the plasma membrane/cell wall and cytoplasm. In MucoRice-CTB, CTB was localized in the cytoplasm and PB-II. In MucoRice-CTB-RNAi, CTB was produced at a level six times that in MucoRice-CTB and was localized, similar to RAG2, in the plasma membrane/cell wall and cytoplasm. Our findings indicate that the relocation of CTB in MucoRice-CTB-RNAi may contribute to down-regulation of RAG2.





Similar content being viewed by others
Abbreviations
- BiP:
-
Binding protein
- CTB:
-
Cholera toxin B-subunit
- DAF:
-
Days after flowering
- IF:
-
Immunofluorescence
- immuno-EM:
-
Immuno-electron microscopy
- RNAi:
-
RNA interference
- PB-I:
-
Protein body I
- PB-II:
-
Protein body II
- PBS:
-
Phosphate-buffered saline
- SDS-PAGE:
-
SDS-polyacrylamide gel electrophoresis
- T-DNA:
-
Transfer DNA
- WB:
-
Western blotting
- WT:
-
Wild type
- Ab:
-
Antibody
- mAb:
-
Monoclonal antibody
- BSA:
-
Bovine serum albumin
- NCS:
-
Newborn calf serum
- PSV:
-
Protein storage vacuole
- ER:
-
Endoplasmic reticulum
- RER:
-
Rough endoplasmic reticulum
- IL-10:
-
Interleukin-10
- PEG:
-
Polyethylene glycol
References
Adachi T, Izumi H, Yamada T, Tanaka K, Takeuchi S, Nakamura R, Matsuda T (1993) Gene structure and expression of rice seed allergenic proteins belonging to the alpha-amylase/trypsin inhibitor family. Plant Mol Biol 21:239–248
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282
Ikezawa Z, Miyakawa K, Komatsu H, Suga C, Miyakawa J, Sugiyama A, Sasaki T, Nakajima H, Hirai Y, Suzuki Y (1992) A probable involvement of rice allergy in severe type of atopic dermatitis in Japan. Acta Derm Venereol Suppl (Stockh) 176:103–107
International Rice Genome Sequencing Project (2005) The map-based sequence of the rice genome. Nature 436:793–800
Katsube T, Kurisaka N, Ogawa M, Maruyama N, Ohtsuka R, Utsumi S, Takaiwa F (1999) Accumulation of soybean glycinin and its assembly with the glutelins in rice. Plant Physiol 120:1063–1074
Kawakatsu T, Takaiwa F (2010) Cereal seed storage protein synthesis: fundamental processes for recombinant protein production in cereal grains. Plant Biotechnol J 8:939–953
Krishnan HB, White JA, Pueppke SG (1992) Characterizaion and location of rice (Oryza sativa L.) seed globulins. Plant Sci 81:1–11
Kumar R, Srivastava P, Kumari D, Fakhr H, Sridhara S, Arora N, Gaur SN, Singh BP (2007) Rice (Oryza sativa) allergy in rhinitis and asthma patients: a clinico-immunological study. Immunobiology 212:141–147
Kuroda M, Kimizu M, Mikami C (2010) A simple set of plasmids for the production of transgenic plants. Biosci Biotechnol Biochem 74:2348–2351
Kurokawa S, Nakamura R, Mejima M, Kozuka-Hata H, Kuroda M, Takeyama N, Oyama M, Satoh S, Kiyono H, Masumura T, Teshima R, Yuki Y (2013) MucoRice-cholera toxin B-subunit, a rice-based oral cholera vaccine, down-regulates the expression of α-amylase/trypsin inhibitor-like protein family as major rice allergens. J Proteome Res 12:3372–3382
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Monzon S, Lombardero M, Perez-Camo I, Saenz D, Lasanta J (2008) Allergic rhinoconjunctivitis after ingestion of boiled rice. J Investig Allergol Clin Immunol 18:487–488
Nakamura R, Matsuda T (1996) Rice allergenic protein and molecular-genetic approach for hypoallergenic rice. Biosci Biotechnol Biochem 60:1215–1221
Nochi T, Takagi H, Yuki Y et al (2007) Rice-based mucosal vaccine as a global strategy for cold-chain- and needle-free vaccination. Proc Natl Acad Sci USA 104:10986–10991
Nochi T, Yuki Y, Katakai Y et al (2009) A rice-based oral cholera vaccine induces macaque-specific systemic neutralizing antibodies but does not influence pre-existing intestinal immunity. J Immunol 183:6538–6544
Saito Y, Shigemitsu T, Yamasaki R, Sasou A, Goto F, Kishida K, Kuroda M, Tanaka K, Morita S, Satoh S, Masumura T (2012) Formation mechanism of the internal structure of type I protein bodies in rice endosperm: relationship between the localization of prolamin species and the expression of individual genes. Plant J 70:1043–1055
Satoh R, Nakamura R, Komatsu A, Oshima M, Teshima R (2011) Proteomic analysis of known and candidate rice allergens between non-transgenic and transgenic plants. Regul Toxicol Pharmacol 59:437–444
Shorrosh BS, Wen L, Zen KC, Huang JK, Pan JS, Hermodson MA, Tanaka K, Muthukrishnan S, Reeck GR (1992) A novel cereal storage protein: molecular genetics of the 19 kDa globulin of rice. Plant Mol Biol 18:151–154
Takahashi H, Saito Y, Kitagawa T, Morita S, Masumura T, Tanaka K (2005) A novel vesicle derived directly from endoplasmic reticulum is involved in the transport of vacuolar storage proteins in rice endosperm. Plant Cell Physiol 46:245–249
Takemoto Y, Coughlan SJ, Okita TW, Satoh H, Ogawa M, Kumamaru T (2002) The rice mutant esp2 greatly accumulates the glutelin precursor and deletes the protein disulfide isomerase. Plant Physiol 128:1212–1222
Tanaka K, Sugimoto T, Ogawa M, Kasai Z (1980) Isolation and characterization of two types of protein bodies in the rice endosperm. Agric Biol Chem 44:1633–1639
Teshima R, Nakamura R, Satoh R (2012) Proteomic and allergenomic analyses of rice and potato proteins. In: Proceedings 3rd international symposium on “Frontiers in Agriculture Proteome Research” pp 124–129
Tokuhara D, Yuki Y, Nochi T, Kodama T, Mejima M, Kurokawa S, Takahashi Y, Nanno M, Nakanishi U, Takaiwa F, Honda T, Kiyono H (2010) Secretory IgA-mediated protection against V. cholerae and heat-labile enterotoxin-producing enterotoxigenic Escherichia coli by rice-based vaccine. Proc Natl Acad Sci USA 107:8794–8799
Tokuhara D, Alvarez B, Mejima M, Hiroiwa T, Takahashi Y, Kurokawa S, Kuroda M, Oyama M, Kozuka-Hata H, Nochi T, Sagara H, Aladin F, Marcotte H, Frenken LG, Iturriza-Gómera M, Kiyono H, Hammarström L, Yuki Y (2013) Rice-based oral antibody fragment prophylaxis and therapy against rotavirus infection. J Clin Invest 123:3829–3838
Trcka J, Schad SG, Scheurer S, Conti A, Vieths S, Gross G, Trautmann A (2012) Rice-induced anaphylaxis: IgE-mediated allergy against a 56-kDa glycoprotein. Int Arch Allergy Immunol 158:9–17
Usui Y, Nakase M, Hotta H, Urisu A, Aoki N, Kitajima K, Matsuda T (2001) A 33-kDa allergen from rice (Oryza sativa L. Japonica). cDNA cloning, expression, and identification as a novel glyoxalase I. J Biol Chem 276:11376–11381
Wakasa Y, Hirano K, Urisu A, Matsuda T, Takaiwa F (2011a) Generation of transgenic rice lines with reduced contents of multiple potential allergens using a null mutant in combination with an RNA silencing method. Plant Cell Physiol 52:2190–2199
Wakasa Y, Yasuda H, Oono Y, Kawakatsu T, Hirose S, Takahashi H, Hayashi S, Yang L, Takaiwa F (2011b) Expression of ER quality control-related genes in response to changes in BiP1 levels in developing rice endosperm. Plant J 65:675–689
Washida H, Sugino A, Doroshenk KA, Satoh-Cruz M, Nagamine A, Katsube-Tanaka T, Ogawa M, Kumamaru T, Satoh H, Okita TW (2012) RNA targeting to a specific ER sub-domain is required for efficient transport and packaging of alpha-globulins to the protein storage vacuole in developing rice endosperm. Plant J 70:471–479
Yamagata H, Tanaka K (1986) The site of synthesis and accumulation of rice storage proteins. Plant Cell Physiol 27:135–145
Yamagata H, Sugimoto T, Tanaka K, Kasai Z (1982) Biosynthesis of storage proteins in developing rice seeds. Plant Physiol 70:1094–1100
Yang L, Hirose S, Takahashi H, Kawakatsu T, Takaiwa F (2012) Recombinant protein yield in rice seed is enhanced by specific suppression of endogenous seed proteins at the same deposit site. Plant Biotechnol J 10:1035–1045
Yuki Y, Byun Y, Fujita M, Izutani W, Suzuki T, Udaka S, Fujihashi K, McGhee JR, Kiyono H (2001) Production of a recombinant hybrid molecule of cholera toxin-B-subunit and proteolipid-protein-peptide for the treatment of experimental encephalomyelitis. Biotechnol Bioeng 74:62–69
Yuki Y, Tokuhara D, Nochi T, Yasuda H, Mejima M, Kurokawa S, Takahashi Y, Kataoka N, Nakanishi U, Hagiwara Y, Fujihashi K, Takaiwa F, Kiyono H (2009) Oral MucoRice expressing double-mutant cholera toxin A and B subunits induces toxin-specific neutralising immunity. Vaccine 27:5982–5988
Yuki Y, Mejima M, Kurokawa S et al (2013) Induction of toxin-specific neutralizing immunity by molecularly uniform rice-based oral cholera toxin B subunit vaccine without plant-associated sugar modification. Plant Biotechnol J 11:799–808
Acknowledgments
We are grateful to Drs. Nana Kawasaki, Kunisuke Tanaka, Koji Kashima, Michiyo Abe, Yoshiko Fukuyama, Yuhi Saito, and Takanari Shigemitsu for useful discussions and technical support. This work was supported by grants from the Programs of Special Coordination Funds for Promoting Science and Technology and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Y.Y., H.K.); the Ministry of Health, Labor and Welfare of Japan (Y.Y., H.K.); the New Energy and Industrial Technology Development Organization (NEDO) (H.K.); the Adaptable and Seamless Technology Transfer Program through Target-driven R&D (A-step) and the Research and Development Program for New Bio-industry Initiatives of the Bio-oriented Technology Research Advancement Institution (Y.Y.).
Conflict of interest
No conflict exists.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Sato.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kurokawa, S., Kuroda, M., Mejima, M. et al. RNAi-mediated suppression of endogenous storage proteins leads to a change in localization of overexpressed cholera toxin B-subunit and the allergen protein RAG2 in rice seeds. Plant Cell Rep 33, 75–87 (2014). https://doi.org/10.1007/s00299-013-1513-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-013-1513-3