Abstract
Key message
The calreticulin triple knockout mutant shows growth defects in response to abiotic stress.
Abstract
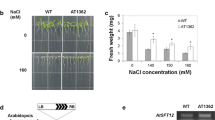
The endoplasmic reticulum (ER) is an essential organelle that is responsible for the folding and maturation of proteins. During ER stress, unfolded protein aggregates accumulate in the cell, leading to the unfolded protein response (UPR). The UPR up-regulates the expression of ER-stress-responsive genes encoding calreticulin (CRT), an ER-localized Ca2+-binding protein. To understand the function of plant CRTs, we generated a triple knockout mutant, t123, which lacks CRT1, CRT2 and CRT3 and examined the roles of calreticulins in abiotic stress tolerance. A triple knockout mutant increased sensitivity to water stress which implies that calreticulins are involved in the Arabidopsis response to water stress. We identified that the cyclophilin AtCYP21-2, which is located in the ER, was specifically enhanced in the t123 mutants. Seed germination of the atcyp21-1 mutant was retarded by water stress. Taken together, these results suggest that regulatory proteins that serve to protect plants from water stress are folded properly in part with the help of calreticulins. The AtCYP21-2 may also participate in this protein-folding process in association with calreticulins.








Similar content being viewed by others
Abbreviations
- 2-DE:
-
2-Dimensional electrophoresis
- ABA:
-
Abscisic acid
- CNX:
-
Calnexins
- CRT:
-
Calreticulin
- DTT:
-
Dithiothreitol
- ER:
-
Endoplasmic reticulum
- IVT:
-
In vitro transcription
- MS:
-
Murashige and Skoog
- PPiase:
-
Peptidyl-prolyl isomerase
- TM:
-
Tunicamycin
- UPR:
-
Unfolded protein response
References
Bergeron JJ, Brenner MB, Thomas DY, Williams DB (1994) Calnexin: a membrane-bound chaperone of the endoplasmic reticulum. Trends Biochem Sci 19:124–128
Brandts JF, Halvorson HR, Brennan M (1975) Consideration of the possibility that the slow step in protein denaturation reactions is due to cis-trans isomerism of proline residues. Biochemistry 14:4953–4963
Chen F, Hayes PM, Mulrooney DM, Pan A (1994) Identification and characterization of cDNA clones encoding plant calreticulin in barley. Plant Cell 6:835–843
Chen MH, Tian GW, Gafni Y, Citovsky V (2005) Effects of calreticulin on viral cell-to-cell movement. Plant Physiol 138:1866–1876
Christensen A, Svensson K, Persson S, Jung J, Michalak M, Widell S, Sommarin M (2008) Functional characterization of Arabidopsis calreticulin 1a: a key alleviator of endoplasmic reticulum stress. Plant Cell Physiol 49:912–924
Christensen A, Svensson K, Thelin L, Zhang W, Tintor N, Prins D, Funke N, Michalak M, Schulze-Lefert P, Saijo Y, Sommarin M, Widell S, Persson S (2010) Higher plant calreticulins have acquired specialized functions in Arabidopsis. PLoS ONE 5(6):e11342
Coughlan SJ, Hastings C, Winfrey RJ (1997) Cloning and characterization of the calreticulin gene from Ricinus communis L. Plant Mol Biol 34:897–911
Denecke J, Carlsson LE, Vidal S, Hoglund AS, Ek B, van Zeijl MJ, Sinjorgo KM, Palva ET (1995) The tobacco homolog of mammalian calreticulin is present in protein complexes in vivo. Plant Cell 7:391–406
Ferreira PA, Nakayama TA, Pak WL, Travis GH (1996) Cyclophilin-related protein RanBP2 acts as chaperone for red/green opsin. Nature 383:637–640
Galat A (1999) Variations of sequences and amino acid compositions of proteins that sustain their biological functions: an analysis of the cyclophilin family of proteins. Arch Biochem Biophys 371:149–162
Gelebart P, Opas M, Michalak M (2005) Calreticulin, a Ca2+-binding chaperone of the endoplasmic reticulum. Int J Biochem Cell Biol 37:260–266
Hammond C, Helenius A (1993) A chaperone with a sweet tooth. Curr Biol 3:884–886
Heilmann I, Shin J, Huang J, Perera IY, Davies E (2001) Transient dissociation of polyribosomes and concurrent recruitment of calreticulin and calmodulin transcripts in gravistimulated maize pulvini. Plant Physiol 127:1193–1203
Hong Z, Jin H, Tzfira T, Li J (2008) Multiple mechanism-mediated retention of a defective brassinosteroid receptor in the endoplasmic reticulum of Arabidopsis. Plant Cell 20:3418–3429
Jia XY, Xu CY, Jing RL, Li RZ, Mao XG, Wang JP, Chang XP (2008) Molecular cloning and characterization of wheat calreticulin (CRT) gene involved in drought-stressed responses. J Exp Bot 59:739–751
Jia XY, Hea LH, Jing RL, Lia RZ (2009) Calreticulin: conserved protein and diverse functions in plants. Physiol Plant 136:127–138
Jin H, Hong Z, Su W, Li J (2009) A plant-specific calreticulin is a key retention factor for a defective brassinosteroid receptor in the endoplasmic reticulum. Proc Natl Acad Sci USA 106:13612–13617
Kamauchi S, Nakatani H, Nakano C, Urade R (2005) Gene expression in response to endoplasmic reticulum stress in Arabidopsis thaliana. FEBS J 272:3461–3476
Komatsu S, Yang G, Khan M, Onodera H, Toki S, Yamaguchi M (2007) Over-expression of calcium-dependent protein kinase 13 and calreticulin interacting protein 1 confers cold tolerance on rice plants. Mol Genet Genomics 277:713–723
Kwiatkowski BA, Zielinska-Kwiatkowska AG, Migdalski A, Kleczkowski LA, Wasilewska LD (1995) Cloning of two cDNAs encoding calnexin-like and calreticulin-like proteins from maize (Zea mays) leaves: identification of potential calcium-binding domains. Gene 165:219–222
Lee H, Guo Y, Ohta M, Xiong L, Stevenson B, Jk Zhu (2002) LOS2, a genetic locus required for cold-responsive gene transcription encodes a bifunctional enolase. EMBO J 21:2692–2702
Leverson JD, Ness SA (1998) Point mutations in v-Myb disrupt a cyclophilin-catalysed negative regulatory mechanism. Mol Cell 1:203–211
Li J, Zhao-Hui C, Batoux M, Nekrasov V, Roux M, Chinchilla D, Zipfel C, Jones JD (2009) Specific ER quality control components required for biogenesis of the plant innate immune receptor EFR. Proc Natl Acad Sci USA 106:15973–15978
Lin DT, Lechleiter JD (2002) Mitochondrial targeted cyclophilin D protects cells from cell death by peptidyl prolyl isomerization. J Biol Chem 277:31134–31141
Martínez IM, Chrispeels MJ (2003) Genomic analysis of the unfolded protein response in Arabidopsis shows its connection to important cellular processes. Plant Cell 15:561–576
McCauliffe DP, Lux FA, Lieu TS, Sanz I, Hanke J, Newkirk MM, Bachinski LL, Itoh Y, Siciliano MJ, Reichlin M, Sontheimer RD, Capra JD (1990) Molecular cloning, expression, and chromosome 19 localization of a human Ro/SS-A autoantigen. J Clin Invest 85:1379–1391
Michalak M, Corbett EF, Mesaeli N, Nakamura K, Opas M (1999) Calreticulin: one protein, one gene, many functions. Biochem J 344:281–292
Nelson DE, Glaunsinger B, Bohnert HJ (1997) Abundant accumulation of the calcium-binding molecular chaperone calreticulin in specific floral tissues of Arabidopsis thaliana. Plant Physiol 114:29–37
Persson S, Wyatt SE, Love J, Thompson WF, Robertson D, Boss WF (2001) The Ca2+ status of the endoplasmic reticulum is altered by induction of calreticulin expression in transgenic plants. Plant Physiol 126:1092–1104
Priyanka B, Sekhar K, Sunita T, Reddy VD, Rao KV (2010) Characterization of expressed sequence tags (ESTs) of pigeon pea (Cajanus cajan L.) and functional validation of selected genes for abiotic stress tolerance in Arabidopsis thaliana. Mol Genet Genomics 283:273–287
Qiu Y, Xi J, Du L, Roje S, Poovaiah BW (2012) A dual regulatory role of Arabidopsis calreticulin-2 in plant innate immunity. Plant J 69:489–500
Romano PG, Horton P, Gray JE (2004) The Arabidopsis cyclophilin gene family. Plant Physiol 134:1268–1282
Saijo Y, Tintor N, Lu X, Rauf P, Pajerowska-Mukhtar K, Häweker H, Dong X, Robatzek S, Schulze-Lefert P (2009) Receptor quality control in the endoplasmic reticulum for plant innate immunity. EMBO J 28:3439–3449
Shieh BH, Stamnes MA, Seavello S, Harris GL, Zuker CS (1989) The NinaA gene required for visual transduction in Drosophila encodes a homolog of cyclosporin A-binding protein. Nature 338:67–70
Smirnoff N (1998) Plant resistance to environmental stress. Curr Opin Plant Biol 9:214–219
Smith MJ (1992a) A C. elegans gene encodes a protein homologous to mammalian calreticulin. DNA Seq 2:235–240
Smith MJ (1992b) Nucleotide sequence of a Drosophila melanogaster gene encoding a calreticulin homologue. DNA Seq 3:247–250
Thelin L, Mutwil M, Sommarin M, Persson S (2011) Diverging functions among calreticulin isoforms in higher plants. Plant Signal Behav 6:905–910
Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P (2000) Functional and genomic analyses reveal essential coordination between the unfolded protein response and ER-associated degradation. Cell 101:249–258
Wang WX, Vinocur B, Shoseyov O, Altman A (2001) Biotechnology of plant osmotic stress tolerance: physiological and molecular considerations. Acta Hortic 560:285–292
Waterhouse NJ, Pinkoski MJ (2007) Calreticulin: raising awareness of apoptosis. Apoptosis 12:631–634
Williams DB (2006) Beyond lectins: the calnexin/calreticulin chaperone system of the endoplasmic reticulum. J Cell Sci 119:615–623
Wilson IB (2002) Glycosylation of proteins in plants and invertebrates. Curr Opin Struct Biol 12:569–577
Wyatt SE, Tsou PL, Robertson D (2002) Expression of the high capacity calcium-binding domain of calreticulin increases bioavailable calcium stores in plants. Transgenic Res 11:1–10
Acknowledgments
This work was supported by a grant from the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (to Hojoung Lee, 2012; grant #2012-112068-3) and by a grant from the National Research Foundation (to Suk-Whan Hong; #2012R1A1A4A01006448).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by K. Chong.
Electronic supplementary material
Below is the link to the electronic supplementary material.

299_2013_1497_Fig9_ESM.jpg
Figure S1. Multiple alignments of amino acid sequences of CRT1, CRT2 and CRT3 proteins. Identical residues are shaded in black (*), and two identical residues are shaded in gray (two dots). CRT1A is CRT1, and CRT1B is CRT2. The CRT1 is At1g56340, CRT2 is At1g09210 and CRT3 is At1g08450. (JPEG 2512 kb)

299_2013_1497_Fig10_ESM.jpg
Figure S2. Growth phenotypes of Arabidopsis wild-type, t12, t13, t23 and t123 mutant seedlings Seven-day-old Arabidopsis wild-type (Col-0), t12, t13, t23 and t123 seedlings grown on normal MS1/2 medium (2 % sucrose) were transferred to soil and allowed to grow for 3 (a) or 7 (b) more weeks before being photographed. The seedlings shown were typical of three separate experiments performed at 23 °C (n = 5-8 seedlings per experiment). (JPEG 1977 kb)

299_2013_1497_Fig11_ESM.jpg
Figure S3. Complementation analysis. A plasmid expressing the CRT1 or CRT3 cDNA under the control of the CRT1 or CRT3 promoter was introduced into the t123 mutant, and 10 independent homozygous transgenic lines were recovered for the complementation analysis shown in the picture. Following sterilization and 3 days of stratification at 4 °C, seeds were germinated in standard MS agar medium supplemented with 2 % sucrose and allowed to grow for 10 days before picture was taken. (JPEG 668 kb)

299_2013_1497_Fig12_ESM.jpg
Figure S4. The expression of CNX1 in Col-0, cyc20-1, cyc21-1, cyc21-2 and t123 seedlings in response to DTT, MG132 or TM Col-0, cyc20-1, cyc21-1, cyc21-2 and t123 mutant seedlings were grown on normal growth medium for two weeks and then transferred to medium containing DTT (10 μM), MG132 (10 μM) or TM (10 μM) for 6 h prior to RNA extraction. The transcript level of CNX1 is shown, and rRNA was used as a loading control. (JPEG 530 kb)

299_2013_1497_Fig13_ESM.jpg
Figure S5. Expression of ATCYP21-2 in Arabidopsis Col-0, t1, t2, t3 and t123 seedlings. (a) Transcription of the Cyclophilin, ATCYP21-2, in 3-week-old Col-1, t1, t2, t3 and t123 plants was determined using qRT-PCR. ACTIN was used as the internal loading control. Ten-day-old seedlings were grown on normal MS1/2 medium before total RNA extraction for RT-PCR. (b) Col-0, t12 and t123 mutant seedlings were grown on normal growth medium for 2 weeks and then transferred to medium containing 300 mM mannitol for 0, 3, 6 or 24 hrs prior to RNA extraction using the ATA reagent. Transcript level of CYP21-2 is shown. rRNA levels were used as loading control. (JPEG 696 kb)
Rights and permissions
About this article
Cite this article
Kim, J.H., Nguyen, N.H., Nguyen, N.T. et al. Loss of all three calreticulins, CRT1, CRT2 and CRT3, causes enhanced sensitivity to water stress in Arabidopsis . Plant Cell Rep 32, 1843–1853 (2013). https://doi.org/10.1007/s00299-013-1497-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-013-1497-z