Abstract
Key message
An efficient, reproducible and genotype-independent in planta transformation has been standardized for sugarcane using seed as explant.
Abstract
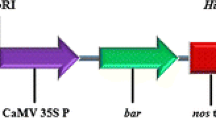
Transgenic sugarcane production through Agrobacterium infection followed by in vitro regeneration is a time-consuming process and highly genotype dependent. To obtain more number of transformed sugarcane plants in a relatively short duration, sugarcane seeds were infected with Agrobacterium tumefaciens EHA 105 harboring pCAMBIA 1304-bar and transformed plants were successfully established without undergoing in vitro regeneration. Various factors affecting sugarcane seed transformation were optimized, including pre-culture duration, acetosyringone concentration, surfactants, co-cultivation, sonication and vacuum infiltration duration. The transformed sugarcane plants were selected against BASTA® and screened by GUS and GFP visual assay, PCR and Southern hybridization. Among the different combinations and concentrations tested, when 12-h pre-cultured seeds were sonicated for 10 min and 3 min vacuum infiltered in 100 µM acetosyringone and 0.1 % Silwett L-77 containing Agrobacterium suspension and co-cultivated for 72-h showed highest transformation efficiency. The amenability of the standardized protocol was tested on five genotypes. It was found that all the tested genotypes responded favorably, though CoC671 proved to be the best responding cultivar with 45.4 % transformation efficiency. The developed protocol is cost-effective, efficient and genotype independent without involvement of any tissue culture procedure and can generate a relatively large number of transgenic plants in approximately 2 months.





Similar content being viewed by others
Abbreviations
- MS:
-
Murashige and Skoog medium
- GA3 :
-
Gibberellic acid
- hpt II:
-
Hygromycin phosphotransferase
- npt II:
-
Neomycin phosphotransferase
- gfp–gus :
-
Green fluorescent protein-β-glucuronidase fusion gene
- CaMV 35S:
-
Cauliflower mosaic virus 35S promoter
References
Adesoye AI, Togun AO, Machuka J (2010) Transformation of cowpea (Vigna unguiculata L. Walp.) by Agrobacterium infiltration. J Appl Biosci 30:1845–1860
Agarwal S, Loar S, Steber C, Zale J (2009) Floral transformation of wheat. Methods Mol Biol 478:105–113
Ahmad M, Mirza B (2005) An efficient protocol for transient transformation of intact fruit and transgene expression in citrus. Plant Mol Biol Rep 23:419a–419k
Ahsan N, Lee SH, Lee DG, Anisuzzaman M, Alam MF, Yoon HS, Choi MS, Yang JK, Lee BH (2007) The effects of wounding type, pre-culture, infection method and co-cultivation temperature on the Agrobacterium-mediated gene transfer in tomatoes. Ann Appl Biol 151:363–372
Akbulut M, Yücel M, Öktem HA (2008) Analysis and optimization of DNA delivery into chickpea (Cicer arietinum L.) seedlings by Agrobacterium tumefaciens. Afr J Biotechnol 7(8):1011–1017
An G (1985) High-efficiency transformation of cultured tobacco cells. Plant Physiol 79:568–570
Arencibia AE, Carmona P, Tellez MT, Chan SM, Yu L, Trujillo, p Oramas (1998) An efficient protocol for sugarcane (Saccharum spp.) transformation mediated by Agrobacterium tumefaciens. Transgenic Res 7:213–222
Arencibia A, Carmona E, Cornide MT, Castiglione S, O’Relly J, Cinea A, Oramas P, Sala F (1999) Somaclonal variation in insect resistant transgenic sugarcane (Saccharum hybrid) plants produced by cell electroporation. Transgenic Res 8:349–360
Arvinth S, Arun S, Selvakesavan RK, Srikanth J, Mukunthan N, Ananda Kumar P, Premachandran MN, Subramonian N (2010) Genetic transformation and pyramiding of aprotinin expressing sugarcane with cry1Ab for shoot borer (Chilo infuscatellus) resistance. Plant Cell Rep 29(4):383–395
Bakshi S, Sadhukhan A, Mishra S, Sahoo L (2011) Improved Agrobacterium-mediated transformation of cowpea via sonication and vacuum infiltration. Plant Cell Rep 30:2281–2292
Bechtold N, Jaudeau B, Jolivet S, Maba B, Vezon D, Voisin R, Pelletier G (2000) The maternal chromosome set is the target of the T-DNA in the in planta transformation of Arabidopsis thaliana. Genetics 155:1875–1887
Bent AF (2000) Arabidopsis in planta transformation. Uses, mechanisms, and prospects for transformation of other species. Plant Physiol 124:1540–1547
Beranová M, Rakousky S, Vávrová Z, Skalicky T (2008) Sonication assisted Agrobacterium-mediated transformation enhances the transformation efficiency in flax (Linux usitatissimum L.). Plant Cell Tissue Org Cult 94:253–259
Bower R, Birch RG (1992) Transgenic sugarcane plants via microprojectile bombardment. Plant J 2:409–416
Cardoza V, Stewart CN Jr (2003) Increased Agrobacterium-mediated transformation and rooting efficiencies in canola (Brassica napus L.) from hypocotyl explants. Plant Cell Rep 21:599–604
Cervera M, Pina JA, Juarez J, Navarro L, Pena L (1998) Agrobacterium-mediated transformation of citrange: factors affecting transformation and regeneration. Plant Cell Rep 18:271–278
Chen X, Equi R, Baxter H, Berk K, Han J, Agarwal S, Zale J (2010) A high-throughput transient gene expression system for switchgrass (Panicum virgatum L.) seedlings. Biotechnol Biofuels 3:9
Cheng M, Fry JE, Pang S, Zhou H, Hironaka CM, Duncan DR, Conner TW, Wan Y (1997) Genetic transformation of wheat mediated by Agrobacterium tumefaciens. Plant Physiol 115:971–980
Chowdhury MKU, Vasil IK (1992) Stably transformed herbicide resistance callus of sugarcane via microprojectile bombardment of cell suspension cultures and electroporation of protoplasts. Plant Cell Rep 11:494–498
Christou P, Ford T, Kofron M (1991) Production of transgenic rice (Oryza sativa L.) plants from agronomically important indica and japonica varieties via electric discharge particle acceleration of exogenous DNA into immature zygotic embryos. Bio/Technology 9:957–962
Chumakov MI, Rozhok NA, Velikov VA, Tyrnov VS, Volokhina IV (2006) Agrobacterium-mediated in planta transformation of maize via pistil filaments. Russ J Genet 42:893–897
Chung MH, Chen MK, Pan SM (2000) Floral spray transformation can efficiently generate Arabidopsis transgenic plants. Transgenic Res 9:471–476
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Curtis IS, Nam HG (2001) Transgenic radish (Raphanus sativus L.var. longipinnatus Bailey) by floral-dip method—plant development and surfactant are important in optimizing transformation efficiency. Transgenic Res 10:363–371
Curtis IS, Power JB, Hedden P, Ward DA, Phillips A, Lower KC, Davey MR (1999) A stable transformation system for the ornamental plant, Datura meteloides (D.C.). Plant Cell Rep 18:554–560
Darbani B, Eimanifar A, Stewart CN, Camargo WN (2007) Methods to produce marker-free transgenic plants. Biotechnol J 2:83–90
De Oliveira MLP, Febres VJ, Costa MGC, Moore GA, Otoni WC (2009) High-efficiency Agrobacterium-mediated transformation of citrus via sonication and vacuum infiltration. Plant Cell Rep 28:387–395
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA mini preparation: version II. Plant Mol Biol Rep 1:19–21
Elliott AR, Campbell JA, Bretell RIS, Grof CPL (1998) Agrobacterium-mediated transformation of sugarcane using GFP as a screenable marker. Aust J Plant Physiol 25:739–743
Enríquez GA, Trujillo LE, Menéndez C, Vázquez-Padrón RI, Tiel K, Dafhnis F, Arrieta J, Selman G, Hernandez L (2000) Sugarcane (Saccharum hybrid) genetic transformation mediated by Agrobacterium tumefaciens: production of transgenic plants expressing proteins with agronomic and industrial value. In: Arencibia A (ed) Developments in plant genetics and breeding. Elsevier, Amsterdam, pp 76–81
Enríquez-Obregón GA, Vázquez-padrón RI, Prieto-sansonov DL, de la Riva GA, Selman-Housein G (1998) Herbicide resistant sugarcane (Saccharum officinarum L.) plants by Agrobacterium-mediated transformation. Planta 206:20–27
FAOSTAT (2011) Agricultural data. http://faostat.fao.org/site/339/default.aspx
Fromm ME, Morrish F, Armstrong C, Williams R, Thomas J, Klein TM (1990) Inheritance and expression of chimeric genes in the progeny of transgenic maize plants. Bio/Technology 8:833–838
Gallo-Meagher M, Irvine JE (1996) Herbicide resistant transgenic sugarcane plants containing the bar gene. Crop Sci 36:1367–1374
Goldberg JB, Ohman DE (1984) Cloning and expression in Pseudomonas aeruginosa of a gene involved with the production of alginate. J Bacteriol 158:1115–1121
Grabowska A, Filipecki M (2004) Infiltration with Agrobacterium—the method for stable transformation avoiding tissue culture. Acta Physiol Plant 26(4):451–458
Hadi MZ, Kemper E, Wendeler E, Reiss B (2002) Simple and versatile selection of Arabidopsis transformants. Plant Cell Rep 21:130–135
Hamza S, Chupeau Y (1993) Re-evaluation of conditions for plant regeneration and Agrobacterium-mediated transformation from tomato (Lycopersicon esculentum). J Exp Bot 44:1837–1845
Herath SP, Suzuki T, Hattori K (2005) Factors influencing Agroacterium-mediated genetic transformation of kenaf. Plant Cell Tissue Org Cult 82:201–206
Hoerlein G (1994) Glufosinate (phosphinothricin), a natural amino acid with unexpected herbicidal properties. Rev Environ Contam Toxicol 138:73–145
Hu CY, Wang L (1999) In-planta soybean transformation technologies developed in China: procedure, confirmation and field performance. In Vitro Cell Dev Biol 35:417–420
Hu Z, Wu YR, Li W, Gao HH (2006) Factors affecting Agrobacterium tumefaciens-mediated genetic transformation of Lycium barbarum L. In Vitro Cell Dev Biol Plant 42:461–466
Huang XQ, Wei ZM (2005) Successful Agrobacterium-mediated genetic transformation of maize elite inbred lines. Plant Cell Tissue Org Cult 83:187–200
Ingelbrecht IL, Irvine JE, Mirkov TE (1999) Post transcriptional gene silencing in transgenic sugarcane. Dissection of homology-dependent virus resistance in a monocot that has a complex polyploid genome. Plant Physiol 119:1187–1197
Jain M, Chengalrayan K, Abouzid A, Gallo M (2007) Prospecting the utility of a PMI/mannose selection system for the recovery of transgenic sugarcane (Saccharum spp. hybrid) plants. Plant Cell Rep 26:581–590
Janssen BJ, Gardner RC (1993) The use of transient GUS expression to develop an Agrobacterium-mediated gene transfer system for kiwi fruit. Plant Cell Rep 12:28–31
Jefferson RA, Kavanagh TA, Bevan NW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Joersbo M, Brunstedt J (1992) Sonication: a new method for gene transfer to plants. Physiol Plant 85:230–234
Kalunke RM, Kolge AM, Babu KH, Prasad DT (2009) Agrobacterium-mediated transformation of sugarcane for borer resistance using Cry 1Aa3 gene and one-step regeneration of transgenic plants. Sugar Tech 11(4):355–359
Keshamma E, Rohini S, Rao KS, Madhusudhan B, Udaya Kumar M (2008) Tissue culture-independent in planta transformation strategy: an Agrobacterium tumefaciens-mediated gene transfer method to overcome recalcitrance in cotton (Gossypium hirsutum L.). J Cotton Sci 12:264–272
Li JF, Park E, von Arnim AG, Nebenführ A (2009a) The FAST technique: a simplified Agrobacterium-based transformation method for transient gene expression analysis in seedlings of Arabidopsis and other plant species. Plant Methods 5:6.A
Li S, Zhao DG, Wu YJ, Tian X (2009b) A simplified seed transformation method for obtaining transgenic Brassica napus plants. Agric Sci China 8(6):658–663
Li J, Tan X, Zhu F, Guo J (2010) Rapid and simple method for Brassica Napus floral-dip transformation and selection of transgenic plantlets. Int J Biol 2:127–131
Lin J, Zhou B, Yang Y, Mei J, Zhao X, Guo X, Huang X, Tang D, Liu X (2009) Piercing and vacuum infiltration of the mature embryo: a simplified method for Agrobacterium-mediated transformation of indica rice. Plant Cell Rep 28:1065–1074
Liu Z, Park B-J, Kanno A, Kameya T (2005) The novel use of a combination of sonication and vacuum infiltration in Agrobacterium-mediated transformation of kidney bean (Phaseolus vulgaris L.) with lea gene. Mol Breed 16:189–197
Liu Y, Yang H, Sakanishi A (2006) Ultrasound: mechanical gene transfer into plant cells by sonoporation. Biotechnol Adv 24:1–16
Lutz KA, Knapp JE, Maliga P (2001) Expression of bar in the plastid genome confers herbicide resistance. Plant Physiol 125:1585–1590
Ma HM, Schulze S, Lee S, Yang M, Mirkov E, Irvine J, Moore P, Paterson A (2004) An EST survey of the sugarcane transcriptome. Theor Appl Genet 108:851–863
Madhou P, Raghavan C, Wells A, Stevenson TW (2006) Genome wide microarray analysis of the effect of a surfactant application in Arabidopsis. Weed Res 46:275–283
Mamontova EM, Velikov VA, Volokhina IV, Chumakov MI (2010) Agrobacterium-mediated in planta transformation of maize germ cells. Russ J Genet 46:501–504
Manickavasagam M, Ganapathi A, Anbazhagan VR, Sudhakar B, Selvaraj N, Vasudevan A, Kasthurirengan S (2004) Agrobacterium-mediated genetic transformation and development of herbicide resistant sugarcane (Saccharum species hybrids) using axillary buds. Plant Cell Rep 23:134–143
Manoj Kumar A, Sreevathsa R, Nanja Reddy K, Ganesh PT, Udayakumar M (2011) Amenability of castor to an Agrobacterium- mediated in planta transformation strategy using a cry1AcF gene for insect tolerance. J Crop Sci Biotech 14(2):125–132
Mariashibu TS, Subramanyam K, Arun M, Mayavan S, Rajesh M, Theboral J, Manickavasagam M, Ganapathi A (2013) Vacuum infiltration enhances the Agrobacterium-mediated genetic transformation in Indian soybean cultivars. Acta Physiol Plant 35:41–54
McHughen A, Jordan M, Feist G (1989) A pre-culture period prior to Agrobacterium inoculation increases production of transgenic plants. J Plant Physiol 135:245–248
Mengiste T, Amedeo P, Paszkowski J (1997) High-efficiency transformation of Arabidopsis thaliana with a selectable marker gene regulated by the T-DNA 19 promoter. Plant J 12:945–948
Molinari HBC, Marur CJ, Daros E, De Campos MKP, De Carvalho JFRP, Filho JCB, Pereira LFP, Vieira LGE (2008) Evaluation of the stress-inducible production of proline in transgenic sugarcane (Saccharum spp.): osmotic adjustment, chlorophyll fluorescence and oxidative stress. Physiol Plant 130(2):218–229
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Naseri G, Sohani MM, Pourmassalehgou A, Allahi S (2012) In planta transformation of rice (Oryza sativa) using thaumatin-like protein gene for enhancing resistance to sheath blight. Afr J Biotechnol 11(31):7885–7893
Park BJ, Liu Z, Kanno A, Kameya T (2005) Transformation of radish (Raphanus sativus L.) via sonication and vacuum infiltration of germinated seeds with Agrobacterium harbouring a group 3 LEA gene from B. Napus. Plant Cell Rep 24:494–500
Pathak MR, Hamzah RY (2008) An effective method of sonication-assisted Agrobacterium-mediated transformation of chickpeas. Plant Cell Tissue Organ Cult 93:65–71
Qing CM, Fan L, Lei Y, Bouchez D, Tourneur C, Yan L, Robaglia C (2000) Transformation of Pakchoi (Brassica rapa L. ssp. chinensis) by Agrobacterium infiltration. Mol Breed 6:67–72
Rao KS, Sreevathsa R, Sharma PD, Keshamma E, Udaya KM (2008) In planta transformation of pigeon pea: a method to overcome recalcitrancy of the crop to regeneration in vitro. Physiol Mol Biol Plant 14:321–328
Rohini VK, Rao KS (2000) Transformation of peanut (Arachis hypogaea L.): a non tissue culture based approach for generating transgenic plants. Plant Sci 150:41–49
Sangwan RS, Bourgeois Y, Sangwan-Norreel BS (1991) Genetic transformation of Arabidopsis thaliana zygotic embryos and identification of critical parameters influencing transformation efficiency. Mol Gen Genet 230:475–485
Sangwan RS, Bourgeois Y, Brown S, Vasseur G, Sangwan-Noreel BS (1992) Characterization of competent cells and early events of Agrobacterium-mediated genetic transformation in Arabidopsis thaliana. Planta 188:439–456
Santarém ER, Trick HN, Essig JS, Finer JJ (1998) Sonication-assisted Agrobacterium-mediated transformation of soybean immature cotyledons: optimization of transient expression. Plant Cell Rep 17:752–759
Sanyal I, Singh AK, Kaushik M, Amla DV (2005) Agrobacterium-mediated transformation of chickpea (Cicer arietinum L.) with Bacillus thuringiensis cry1Ac gene for resistance against pod borer insect Helicoverpa armigera. Plant Sci 168:1135–1146
Seol E, Jung Y, Lee J, Cho C, Kim T, Rhee Y, Lee S (2008) In planta transformation of Notocactus scopa cv. Soonjung by Agrobacterium tumefaciens. Plant Cell Rep 27:1197–1206
Smith RH, Hood EE (1995) Review and interpretation: Agrobacterium tumefaciens transformation of monocotyledons. Crop Sci 35:301–309
Solís JIF, Mlejnek P, Studená K, Procházka S (2003) Application of sonication-assisted Agrobacterium-mediated transformation in Chenopodium rubrum L. Plant Soil Environ 49(6):255–260
Spolaore S, Trainotti L, Casadoro G (2001) A simple protocol for transient gene expression in ripe fleshy fruit mediated by Agrobacterium. J Exp Bot 52:845–850
Sreeramanan M, Maziah M, Abdullah MP, Rosli NM, Xavier R (2006) Potential selectable marker for genetic transformation in banana. Biotechnology 5:189–197
Sriskandarajah S, Goodwin P (1998) Conditioning promotes regeneration and transformation in apple leaf explants. Plant Cell Tissue Organ Cult 53:1–11
Stachel SE, Messens E, Van Montagu M, Zambryski PC (1985) Identification of the signal molecules produced by wounded plant cells that activate T-DNA transfer in Agrobacterium tumefaciens. Nature 318:624–629
Subramanyam K, Subramanyam K, Sailaja KV, Srinivasulu M, Lakshmidevi K (2011) Highly efficient Agrobacterium-mediated transformation of banana cv. Rasthali (AAB) via sonication and vacuum infiltration. Plant Cell Rep 30:425–436
Supartana P, Shimizu T, Shioiri H, Nogawa M, Nozue M, Kojima M (2005) Development of simple and efficient in planta transformation method for rice (Oryza zativa L.) using Agrobacterium tumefaciens. J Biosci Bioeng 100:391–397
Supartana P, Shimizu T, Nogawa M, Shioiri H, Nakajima T, Haramoto N, Nozue M, Kojima M (2006) Development of simple and efficient in planta transformation method for wheat (Triticum aestivum L.) using Agrobacterium tumefaciens. J Biosci Bioeng 100:391–397
Suprasanna P, Patade VY, Desai NS, Devarumath RM, Kawar PG, Pagariya MC, Ganapathi A, Manickavasagam M, Babu KH (2011) Biotechnological developments in sugarcane improvement: an overview. Sugar Tech 13(4):322–335
Tachibana K, Watanabe T, Sekizawa T, Takematsu T (1986) Action mechanism of bialaphos II: accumulation of ammonia in plants treated with bialaphos. J Pest Sci 11:33–37
Tague BW, Mantis J (2006) In planta Agrobacterium-mediated transformation by vacuum infiltration. Methods Mol Biol 323:215–223
TianZi C, ShenJie Wu, Jun Z, WangZhen G, TianZhen Z (2010) Pistil drip following pollination: a simple in planta Agrobacterium-mediated transformation in cotton. Biotechnol Lett 32:547–555
Trick HN, Finer JJ (1997) SAAT: sonication-assisted Agrobacterium-mediated transformation. Transgenic Res 6:329–337
Trieu AT, Burleigh SH, Kardailsky IV, Maldonado-Mendoza IE, Versaw WK, Blaylock LA, Shin H, Chiou TJ, Katagi H, Dewbre GR, Weigel D, Harrison MJ (2000) Transformation of Medicago truncatula via infiltration of seedlings or flowering plants with Agrobacterium. Plant J 22:531–541
Trifonova A, Madsen S, Olesen A (2001) Agrobacterium-mediated transgene delivery and integration into barley under a range of in vitro culture conditions. Plant Sci 161:871–880
Valdez-Ortiz A, Medina-Godoy S, Elena Valverde M, Paredes-Lόpez O (2007) A transgenic tropical maize line generated by the direct transformation of the embryo-scutellum by A. tumefaciens. Plant Cell Tissue Organ Cult 91:201–214
Vasil V, Castillo AM, Fromm EM, Vasil IK (1992) Herbicide resistant transgenic wheat plants obtained by microprojectile bombardment of regenerable embryogenic callus. Bio/Technology 10:667–674
Weeks JT, Ye J, Rommens CM (2008) Development of an in planta method for transformation of alfalfa (Medicago sativa). Transgenic Res 17(4):587–597
Weng LX, Deng HH, Xu JL, Li Q, Zhang YQ, Jiang ZD, Li QW, Chen JW, Zhang LH (2011) Transgenic sugarcane plants expressing high levels of modified cry1Ac provide effective control against stem borers in field trials. Transgenic Res 20:759–772
Wu LG, Birch RG (2007) Doubled sugar content in sugarcane plants modified to produce a sucrose isomer. Plant Biotech J 51:109–117
Wu H, Sparks C, Amoah B, Jones HD (2003) Factors influencing successful Agrobacterium-mediated genetic transformation of wheat. Plant Cell Rep 21:659–668
Yasmeen A, Mirza B, Inayatullah S, Safdar N, Jamil M, Ali S, Choudhry MF (2009) In planta transformation of tomato. Plant Mol Biol Rep 27(1):20–28
Zhang L, Xu J, Birch RG (1999) Engineered detoxification confers resistance against a pathogenic bacterium. Nat Biotechnol 17:1021–1024
Zhangsun DT, Luo SL, Chen RK, Tang KX (2007) Improved Agrobacterium-mediated genetic transformation of GNA transgenic sugarcane. Biologia 62(4):386–393
Acknowledgments
The authors are grateful to the University Grants Commission (UGC), Government of India, for the financial support [No.F. 2-3/2011 (Policy/SR)] to carry out the present work. The corresponding author is thankful to University Grants Commission (UGC), Govt. of India, for providing fellowship under UGC-BSR scheme. Subramanyam and Sivanadhan are thankful to the Council of Scientific and Industrial Research (CSIR), Govt. Of India, for the award of Senior Research Fellowship (SRF) for their doctoral research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Lakshmanan.
Rights and permissions
About this article
Cite this article
Mayavan, S., Subramanyam, K., Arun, M. et al. Agrobacterium tumefaciens-mediated in planta seed transformation strategy in sugarcane. Plant Cell Rep 32, 1557–1574 (2013). https://doi.org/10.1007/s00299-013-1467-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-013-1467-5