Abstract
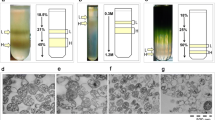
A valuable method to isolate and purify mitochondria from embryonal masses of two coniferous species (Picea abies [L.] Karst. and Abies cephalonica Loud.) is described. Crude mitochondria from both species were shown to be intact, oxygen consuming (with malate plus glutammate, succinate and NADH as substrates) and well coupled (respiratory control ratio ca. 4). The oxidation of the substrates was only partially KCN-insensitive (alternative oxidase) in some cases. However, these fractions were contaminated by membranes (e.g. plasmalemma, tonoplast, Golgi and endoplasmic reticulum). After purification by a discontinuous Percoll gradient (18, 23, 40%, v/v), three mitochondrial populations were separated. The 0/18 interface fraction was composed mainly of broken and uncoupled mitochondria, while the other two (18/23 and 23/40 interface fractions) contained intact and coupled mitochondria, but only 23/40 interface fraction revealed to be better purified starting from both coniferous embryonal masses. In the latter purified fraction, the presence of a cyclosporin A-sensitive K +ATP channel was demonstrated. These findings were discussed in the light of the potential use of these mitochondrial fractions in bioenergetic studies, or in the involvement of these organelles to stress response in conifers.





Similar content being viewed by others
Abbreviations
- BSA:
-
Bovine serum albumin
- DTE:
-
Dithioerythritol
- EDTA:
-
Ethylenediaminetetraacetic acid
- mΔΨ:
-
Mitochondrial electrical potential
- PCD:
-
Programmed cell death
- PEMs:
-
Pro-embryogenic cell masses
- PVPP:
-
Polyvinylpolypyrrolidone
- SE:
-
Somatic embryogenesis
References
Amirsadeghi S, Robson CA, Vanlerberghe GC (2007) The role of the mitochondrion in plant responses to biotic stress. Physiol Plant 129:253–266
Baehrecke EH (2005) Autophagy: dual roles in life and death? Nature Rev Mol Cell Biol 6:505–510
Bellin Mariano A, Kovalhuk L, Valente C, Maurer-Menestrina J, Pereira-Netto AB, Guerra MP, Skare Carnieri EG (2004) Improved method for isolation of coupled mitochondria of Araucaria angustifolia (Bert.) O.Kuntze. Brazil Arch Biol Technol 47:873–879
Bozhkov PV, Filonova LH, Suarez MF, Helmersson A, Smertenko AP, Zhivotovsky B, Von Arnold S (2004) Veidase is a principal caspase-like activity involved in plant programmed cell death and essential for embryonic pattern formation? Cell Death Differ 11:175–182
Bozhkov PV, Filonova LH, Von Arnold S (2002) A key developmental switch during Norway spruce somatic embryogenesis is induced by withdrawal of growth regulators and is associated with cell death and extracellular acidification. Biotech Bioengin 77:658–667
Bozhkov PV, Filonova LH, Suarez MF (2005) Programmed cell death in plant embryogenesis. Curr Top Dev Biol 67:135–179
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Casolo V, Petrussa E, Krajňáková J, Macrì F, Vianello A (2005) Involvement of the mitochondrial K +ATP channel in H2O2 or NO-induced programmed death of soybean suspension cell cultures. J Exp Bot 56:997–1006
Cornu S, Pireaux J-C, Gerard J, Dizengremel P (1996) NAD(P)+-dependent isocitrate dehydrogenase in mitochondria purified from Picea abies seedlings. Physiol Plant 96:312–318
Cross JM, Briggs R, Dohormann VC, Rayle PM (1978) Auxin receptors of maize coleoptile membranes do not have ATPase activity. Plant Physiol 61:242–250
de Virville JD, Aaron I, Alin M-F, Moreau F (1994) Isolation and properties of mitochondria from Arabidopsis thaliana cell suspension cultures. Plant Physiol Biochem 32:159–166
Dong JZ, Dunstan DI (2000) Molecular biology of somatic embryogenesis in conifers. In: Jain SM, Minocha SC (eds) Molecular biology of woody plants, vol 1. Kluwer Academic, Dordrecht, pp 51–88
Douce R, Bourguignon J, Brouquisse R, Neuburger M (1987) Isolation of plant mitochondria: general principles and criteria of integrity. Meth Enzymol 148:403–414
El Meskaoui A, Desjardins Y, Tremblay FM (2000) Kinetics of ethylene biosynthesis and its effects during maturation of white spruce somatic embryos. Physiol Plant 109:333–342
Filonova LH, Bozhkov PV, Brukhin VB, Daniel G, Zhivotovsky B, Von Arnold S (2000a) Two waves of programmed cell death occur during formation and development of somatic embryos in the Gymnosperm, Norway spruce. J Cell Sci 113:4399–4411
Filonova LH, Bozhkov PV, Von Arnold S (2000b) Developmental pathway of somatic embryogenesis in Picea abies as revealed by time-lapse tracking. J Exp Bot 51:249–264
Gupta PK, Durzan DJ (1986) Somatic polyembryogenesis from callus of mature sugar pine embryos. Biotechnol 4:643–645
Halliwell B (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life Plant Physiol 141:312–322
Joy RW, Vogel HJ, Thorpe TA (1997) Inorganic nitrogen metabolism in embryogenic white spruce cultures: a nitrogen 14/15 NMR study. J Plant Physiol 151:306–315
Klimaszewska K, Morency F, Jones-Overton C, Cooke J (2004) Accumulation pattern and identification of seed storage proteins in zygotic embryos of Pinus strobus and in somatic embryos from different maturation treatments. Physiol Plant 121:682–690
Krajňáková J, Gömöry D, Häggman H (2007) Somatic embryogenesis in Abies cephalonica. Can J For Res (accepted)
Kumar R, Lelu M-A, Small I (1995) Purification of mitochondria and mitochondrial nucleic acids from embryogenic suspension cultures of a gymnosperm, Larix x leptoeuropaea. Plant Cell Rep 14:534–538
Kuo J-L, Huang H-J, Cheng C-M, Chen L-J, Huang B-L, Huang L-C, Kuo T-T (1995) Rejuvenation in vitro: modulation of protein phosphorylation in Sequoia sempervirens. J Plant Physiol 146:333–336
Lam E 2004) Controlled cell death, plant survival and development. Nature Rev Mol Cell Biol 5:305–315
Lipavská H, Konrádová H (2004) Somatic embryogenesis in conifers: the role of carbohydrate metabolism. In Vitro Cell Dev Biol Plant 40:23–30
Lord JM (1983) Endoplasmic reticulum and ribosomes. In: Hall JL, Moore AL (eds) Isolation of membranes and organelles from plant cells. Academic, London, p 131
Mannella CA (2000) Introduction: our changing views of mitochondria. J Bioenerg Biomembr 32:1–4
Minocha R, Minocha SC, Long S (2004) Polyamines and their biosynthetic enzymes during somatic embryo development in red spruce (Picea rubens Sarg.). In Vitro Cell Dev Biol Plant 40:572–580
Minocha R, Smith DR, Reeves C, Steele KD, Minocha SC (1999) Polyamine levels during the development of zygotic and somatic embryos of Pinus radiata. Physiol Plant 105:155–164
Møller IM (2001) Plant mitochondrial and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxigene species. Annu Rev Plant Physiol Plant Mol Biol 52:561–591
Møller IM, Lin W (1986) Membrane-bound NAD(P)H dehydrogenases in higher plant cells. Annu Rev Plant Physiol 37:307–334
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:493–497
Obara K, Kuriyama H, Fukuda H (2001) Direct evidence of active and rapid nuclear degradation triggered by vacuole rupture during programmed cell death in Zinnia. Plant Physiol 125:615–626
Pastore D, Stoppelli MC, Di Fonzo N, Passarella S (1999) The existence of the K+ channel in plant mitochondria. J Biol Chem 274:26683–26690
Pastore D, Trono D, Laus MN, Di Fonzo N, Flagella Z (2007) Possible plant mitochondria involvment in cell adaptation to drought stress. A case study durum wheat mitochondria. J Exp Bot 58:195–210
Petrussa E, Casolo V, Braidot E, Chiandussi E, Macrì F, Vianello A (2001) Cyclosporin A induces the opening of a potassium-selective channel in higher plant mitochondria. J Bioenerg Biomembr 33:107–117
Smertenko AP, Bozhkov PV, Filonova LH, Von Arnold S, Hussey PJ (2003) Re-organisation of the cytoskeleton during developmental programmed cell death in Picea abies embryos. Plant J 33:813–824
Stasolla C, Kong L, Yeung EC, Thorpe TA (2002) Somatic embryogenesis in conifers: morphogenesis, physiology, biochemistry, and molecular biology. In Vitro Cell Dev Biol Plant 38:93–105
Stasolla C, Van Zyl L, Egertsdotter U, Craig D, Liu WB, Sederoff RR (2003) The effects of polyethylene glycol on gene expression of developing white spruce somatic embryos. Plant Physiol 131:49–60
Stasolla C, Yeung E (2003) Recent advances in conifer somatic embryogenesis: improving somatic embryos quality. Plant Cell Tissue Organ Cult 74:15–35
Stasolla C, Yeung EC (1999) Ascorbic acid improves conversion of white spruce somatic embryos. In Vitro Cell Dev Biol Plant 35:316–319
Stasolla C, Yeung EC (2001) Ascorbic acid metabolism during white spruce somatic embryo maturation and germination. Physiol Plant 111:196–205
Suarez MF, Filonova LH, Smertenko A, Savenkov EI, Clapham DH, von Arnold S, Zhivotovsky B, Bozhkov PV (2004) Metacaspase dependent programmed cell death is essential for plant embryogenesis. Curr Biol 14: R339–R340
Tolbert NE, Oeser A, Kisaki T, Hageman RH, Yamazaki RK (1968) Peroxisomes from spinach leaves containing enzymes related to glycolate metabolism. J Biol Chem 243:5179–5184
Vianello A, Zancani M, Peresson C, Petrussa E, Casolo V, Krajnakova J, Patui S, Braidot E, Macri F (2007) Plant mitochondrial pathway leading to programmed cell death. Physiol Plant 129:242–252
von Arnold S, Sabala I, Bozhkov P, Dyachok J, Filonova L (2002) Developmental pathways of somatic embryogenesis. Plant Cell Tissue Organ Cult 69:233–249
Acknowledgment
This research was supported by the University of Udine.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Jouanin.
Rights and permissions
About this article
Cite this article
Petrussa, E., Bertolini, A., Krajňáková, J. et al. Isolation of mitochondria from embryogenic cultures of Picea abies (L.) Karst. and Abies cephalonica Loud.: characterization of a K +ATP channel. Plant Cell Rep 27, 137–146 (2008). https://doi.org/10.1007/s00299-007-0436-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-007-0436-2