Abstract
A current priority of vaccinology is the development of multicomponent vaccines that protect against several pathogens. The diphtheria–pertussis–tetanus (DPT) vaccine prevents the symptoms of three serious and often fatal diseases due to the exotoxins produced by Corynebacterium diphteriae, Bordetella pertussis and Clostridium tetani. We are attempting to develop an edible DPT multicomponent vaccine in plants, based on the fusion of protective exotoxin epitopes encoded by synthetic genes. By means of Agrobacterium mediated transformation we generated transgenic tomatoes with a plant-optimised synthetic gene encoding a novel polypeptide containing two adjuvant and six DPT immunoprotective exotoxin epitopes joined by peptide linkers. In transformed tomato plants, integration of the synthetic DPT (sDPT) gene detected by PCR was confirmed by Southern blot, and specific transcripts of the expected molecular size were detected by RT-PCR. Expression of the putative polypeptide encoded by the sDPT gene was detected by immunoassay with specific antibodies to the diphtheria, pertussis and tetanus exotoxins. The sDPT gene is therefore integrated, transcribed and translated as the expected recombinant sDPT multiepitope polypeptide in transgenic tomatoes that constitute a potential edible vaccine.
Similar content being viewed by others
Introduction
The diphtheria–pertussis–tetanus (DPT) vaccine, used to immunize against diphtheria, pertussis (whooping cough) and tetanus has dramatically cut down the risks of these diseases and the consequent deaths in children (Gerathy 1984; Hinman and Koplan 1984). Commercially available DPT vaccines are composed of toxoids of Corynebacterium diphteriae and Clostridium tetani; the pertussis portion is composed of killed Bordetella pertussis bacteria, whose toxins are responsible for the neurological complications of the disease and the secondary effects of the vaccine (Koplan et al. 1979; Cavanaugh et al. 1981). Since its development in the mid 1930s and its widespread use by the late 1950s there have been repeated reports of children reacting to the pertussis vaccine with fever, drowsiness and in rare cases convulsions, mental retardation, learning disabilities and physical handicaps, though rare, these reactions may cause some to resist vaccination (Baraff et al. 1983; Meszaros et al. 1996).
Current vaccine development efforts are focused to produce subunit vaccines which are pathogen-derived proteins (or just an immunogenic domain of a protein, i.e., an epitope) that cannot cause disease but elicit protective immune responses against the corresponding pathogens. Subunit vaccines are generally safer to produce (by eliminating the need to culture pathogenic organisms) and more importantly, to use (for example, see Yu and Langridge 2001). Despite their efficacy subunit vaccines currently depend on capital-intensive fermentation-based technology and the cold chain for their delivery, two factors which limit their use in the developing world, where they are most needed (Rigano and Walmsley 2005).
With the advent of molecular biology techniques in the 1980s, new strategies were developed to generate subunit vaccines (Moffat 1995; Daniell et al. 2001). Production of antigens in genetically-engineered plants could provide an inexpensive source of edible vaccines and antibodies to help fight infectious diseases such as rabies, cholera, hepatitis B, rotavirus, human papillomavirus and AIDS (McGarvey et al. 1995; Thanavala et al. 1995; Kim and Langridge 2003; Jani et al. 2004; Kim et al. 2004).
Transgenic plants are showing considerable potential for the economic production of proteins, with a few already being marketed (Horn et al. 2004). Clinical trials of pharmaceuticals produced in transgenic plants are encouraging the use of this delivery system (Haq et al. 1995; Tacket et al. 1998). However, foreign proteins usually accumulate at relatively low levels in plants (0.01–2% of total soluble protein). Techniques to enhance recombinant antigenic protein accumulation in plant tissues are being explored, such as optimisation of the coding sequence of bacterial or viral genes for optimal expression and targeting to the subcellular compartment in which they accumulate at higher levels in addition transgenic plants can be scaled up for manufacturing purposes by just planting more acreage (as reviewed by Daniell et al. 2001 and Fischer and Emans 2000). The world’s first regulatory approval for a plant cell-made vaccine on a culture based approach against Newcastle disease virus for veterinary use, recently granted to Dow AgroScience represents a milestone in the process of plant-vaccine based research (http://www.news.ft.com/), however it is not yet available in the market and will take 2–3 years for it.
In this paper we describe the generation of transgenic tomato plants expressing an optimised synthetic gene encoding a polypeptide with epitopes of the DPT exotoxins which maintain their antigenicity without secondary effects. These plants are currently being tested as potential edible DPT subunit vaccines.
Materials and methods
Chemicals were purchased from Sigma Chemical Co., St Louis MO; PhytoTechnology Lab., Shawnee Mission, KS; USB Co., Cleveland Ohio; Roche Co., Mannheim; Invitrogen Co., Carlsbad, CA; Promega Co., Madison, WI; Stratagene, La Jolla, CA; Qiagen Inc, Valencia, CA; unless otherwise stated on the text.
Vector construction
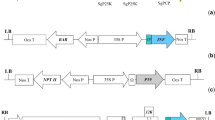
Codon usage for the sDPT gene sequences of the C. diphteriae, B. pertussis and C. tetani exotoxins was modified as per the codon bias for tomato genes, maintaining the original amino acid sequence of the wild type bacterial genes (http://www.kasuza.or.jp/codon/); mRNA processing and destabilising motifs were eliminated using the program back translation tool (http://www.entelechon.de/index.php?id=tools/backtranslation&lang=eng). The sequence encoding the SEKDEL endoplasmic retention signal was added at the 3′ end of the sDPT gene. The 570 bp sequence containing a BamHI site at the 5′ end and a SacI site at the 3′ end was synthesised and cloned by Entelechon (Regensburg, Germany) in the pCR4TOPO vector, and digested with BamHI and SacI to release the insert. The sDPT coding sequence was subcloned by replacing the uidA gene in the binary vector pBI121 (Stratagene) which had been digested with the same enzymes. The resulting plasmid was designated pBI-DPT (Fig. 1). A positive clone was selected by restriction analysis and the plasmid was then mobilised into Agrobacterium tumefaciens LBA 4404 cells via electroporation (according to manufacturer’s recommendations). Positive LBA4404 transformants were verified by restriction analysis and used for plant transformation. All procedures were performed using standard techniques (Sambrook et al. 1989).
Schematic representation of the pBI-DPT construct. The construct contains the synthetic DPT (sDPT) gene formed by coding sequences for the immunoprotective epitopes of the C. diphtheriae, B. pertussis and C. tetani exotoxins under the control of the 35SCaMV promoter. Adjuvant encoding sequences and the SEKDEL endoplasmic retention signal are located at the 3′ end of the gene with the indicated restriction enzyme sites for subcloning in the pBI121 binary vector. DPT-F and DPT-R primers locations are indicated
Plant transformation and regeneration
The procedure was based on the report of Cortina and Culiañez-Macià (2004).
Tomato (Lycopersicon esculentum cv AC) seeds were surface-sterilised by soaking for 10 min in 50% sodium hypochlorite commercial bleaching solution. Seeds were then washed five times in sterile water, germinated in Petri dishes containing MS tissue culture medium (Murashige and Skoog 1962) (4.6 g l−1), supplemented with sucrose (30 g l−1) and agar (8 g l−1) at pH 5.7, and grown at 25°C on a photoperiod of 16-h light/ 8-h dark cycle. After 1 week cotyledons of the tomato seedlings were cut off and the tips were removed and sectioned transversely with a scalpel in two fragments. Cotyledon pieces were placed with the abaxial side facing up in Petri dishes containing preculture medium (MS salts, 30 g l−1 sucrose, 8 g l−1 agar, 1 mg l−1 naphthalene acetic acid (NAA) and 1 mg l−1 6-benzylaminopurine) and incubated for 2 days at 25°C in the dark.
A. tumefaciens LBA4404 cells harbouring the binary vector pBI-DPT with the neomycin phosphotransferase (nptII) gene as selection marker were grown to 0.2–0.3 OD600 in YM medium for 2 days prior to co-cultivation. Tomato cotyledon explants were removed from preculture medium and transferred to the bacterial suspension in YM medium for 15 min; cotyledon explants were then placed onto preculture agar plates for 2 days at 25°C in the dark, ensuring explants were in contact with the agar surface.
After 2 days cotyledon segments were immersed in the washing medium (MS salts, 30 g l−1 sucrose, 300 mg l−1 cefotaxime), blotted dry on sterile filter paper and transferred to shoot regeneration medium (MS salts, 30 g l−1 sucrose, 8 g l−1 agar, 1.75 mg l−1 zeatin, 0.87 mg l−1 IAA, 300 mg l−1 cefotaxime, 100 mg l−1 kanamycin monosulphate). Four months later, when regenerated plantlets reached 2–3 cm in height, they were separated from calli and placed on a rooting medium (MS salts, 300 mg l−1 cefotaxime, 100 mg l−1 kanamycin monosulphate) in sterile plant containers. After 1 month in rooting medium, the plants were transferred to soil in an environmental chamber until they reached the adult stage and set fruits.
PCR analysis of transgenic plants
Genomic DNA was isolated from leaves as described by Dellaporta et al. (1983). The presence of the sDPT gene was determined by PCR analysis using the specific primers DPT-F (5′-ATGCACCATCACCACCATC-3′) and DPT-R (5′-GCAACTCATCTTTTTCACTCTCG-3′). Genomic DNA (200 ng) was used as template in PCR mixtures containing 2.5 mM MgCl2, 0.2 mM dNTPs, 0.5 μM of each primer and 0.5 units of Taq DNA polymerase. Cycling conditions were 94°C for 30 s, 60°C for 30 s and 72°C for 45 s for 35 cycles. Amplifications were performed in a Techne thermocycler (New Jersey, NJ, USA). PCR products were analysed by electrophoresis in 1.5% agarose gels. Ten nanogram of pBI-DPT were used in positive PCR control mixtures.
Southern blot analysis
Genomic DNA was isolated from leaves of tomato plants as described by Dellaporta et al. (1983). Forty micrograms of genomic DNA from wild type and transgenic plants were digested with EcoRI to determine the transgene copy number. Digested DNA was electrophoresed in 1% agarose gels and blotted on Hybond N membranes (Amersham, Buckinghamshire, UK) following standard procedures (Sambrook et al. 1989). A DIG-labelled probe was generated with the PCR DIG Labelling Mix (Roche Co., Mannheim, Germany). Hybridisation was performed using PCR DIG High Prime DNA Labelling mix using the clone with sDPT and sDPT primers above mentioned and Detection Starter Kit II (Roche Applied Science, Mannheim, Germany) following the supplier’s instructions.
RT-PCR analysis
Total RNA from leaves was extracted with TRIZOL (Invitrogen, Carlsbad, CA, USA) according to the supplier’s instructions. Two-hundred-and-fifty nanograms of total RNA was reverse transcribed with the MultiScribe Reverse Transcriptase system (Applied Biosystems, Foster City, CA, USA) with random 6-mer primers in 10-μl reaction mixtures. Amplification of cDNA was carried out for 10 min at 25°C, 60 min at 37°C and 5 min at 95°C. Aliquots of 2 μl cDNA were used for each PCR mix using the sDPT gene primers. Amplified products were subjected to electrophoresis in 1.5% agarose gels. Amplified actin-mRNA was included as a quality control in ethidium bromide stained gels.
Protein extraction and ELISA assays
Tomato leaves or fruit (300 mg) were ground in liquid nitrogen and extracted at 4°C with 400 μl PBS (100 mM NaCl, 10 mM Na2HPO4, 3 mM KH2PO4, pH 7.2) supplemented with 0.5% Triton X-100 and 10 g ml−1 leupeptin. Extracts were centrifuged for 20 min at 13,000×g. Total protein was quantified with the Bradford (1979) assay. sDPT antigen levels were analysed by ELISA in 96-well Plates (Nunc-Immuno Plate, Brand, USA) loaded with soluble protein leaf or fruit extracts from transgenic and wild type plants in coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6).
Titration of the C. tetani, C. diphteriae and B. pertussis exotoxin epitopes was performed using the following specific antisera: anti-tetanus toxin (1:2,000 dilution; USBiological T2962–07, Swampscott, MA, US), anti-diphtheria toxin (1:5,000 dilution; USBiological 8065–51, Swampscott, MA, US) and anti-pertussis toxin (1:400 dilution; Lee et al. 2002). Washed plates containing bound antigen–antibody complexes were incubated with mouse anti-goat alkaline phosphatase conjugate (1:20,000 dilution). The plates were then incubated with fresh of p-nitrophenyl phosphate (1 mg ml−1) for 30 min and read at 450 nm in a model 550 microplate reader (Bio Rad, Hayward, CA, US). Protein content in leaves and fruits of transformed plants was estimated in microgram per gram fresh weight (μg g−1) for the three epitopes.
Results
Design and cloning of the synthetic DPT (sDPT) gene
Tetanus toxin is composed of two subunits, the heavy (H) and light (L) chains, with three functional domains. Domain HC or fragment C (TetC) is a good candidate for a subunit vaccine because it is atoxic, highly immunogenic and induces a protective response (Ribas et al. 2000; Tregoning et al. 2005). Pertussis toxin (PT) is one of the most important virulence factors of B. pertussis that has been used in the acellular pertussis vaccine formulation. PT is composed of five subunits, among which the S1 subunit is immunodominant; antibodies to it have been shown to neutralize the toxin in vitro and to protect mice against live B. pertussis challenge (Boucher et al. 1994; Lee et al. 2002). Diphtheria toxin comprises the A subunit with the catalytic (C) domain, and the B subunit which includes the transmembrane and receptor-binding domains. The C domain participates in membrane interactions and translocation, making it a candidate for subunit vaccine production (Tortorella et al. 1995; Lobeck et al. 1998).
Based on the knowledge of the DPT subunit vaccine candidates, two of the most antigenic immunoprotective epitopes of the diphtheria, pertussis and tetanus exotoxins were chosen by us to design a multiepitope polypeptide using the Antheprot software (available at http://www.antheprot-pbil.ibcp.fr); linker peptide sequences were added to keep the epitope sequences exposed. The following linear antigenic peptide sequences were selected: 26 amino acids (positions 312–337) and the 32 amino acids (positions 975–826) of the tetanus toxin heavy chain, the first 17 amino acids and 17 additional amino acids (positions 99–115) of the pertussis toxin subunit 1, 16 amino acids (positions 186–201) and 27 additional amino acids (position 221–247) of the diphtheria toxin subunit A. Two adjuvant-coding sequences of the tetanus toxin were added near the C-terminal before the endoplasmic retention signal SEKDEL. Codon optimisation was achieved without changing the amino acid sequences of the native exotoxins. 66% of the native codon sequence was changed in the optimization process.
The CaMV 35 S promoter and the Nos terminator were used to regulate the expression of the coding sequence and the nptII gene was used as a selection marker. Restriction sites for BamHI and SacI were introduced at the 5′ and 3′ ends, respectively, for convenient cloning into plant expression vectors. The binary pBI-DPT vector (Fig. 1) was obtained as described in experimental procedures and used for tomato transformation.
Transgenic tomato plants containing the sDPT gene
The pBI-DPT vector was introduced into A. tumefaciens bacteria which were co-cultivated with tomato cotyledon explants to generate transgenic plants via somatic organogenesis. Putative transformed cotyledons were initially cultured with kanamycin in consecutive rounds and the selected individuals were tested by PCR for the presence of the sDPT gene. The primer oligonucleotide set DPT-F/DPT-R was designed and used to detect the foreign gene in tomato tissues. Analysis showed an amplified product of the expected size (570 bp) in all plants transformed with the pBI-DPT vector but not in wild type plants (Fig. 2). After four months in co-culture medium and one month in rooting medium, PCR-positive plants were transferred to soil and allowed to reach the adult stage. Transgenic plants were phenotypically normal (data not shown).
Southern blot analysis and sDPT transgene transcripts
Genomic DNA from tomato plants was digested with EcoRI which cuts once within the T-DNA to determine transgene copy number. Hybridisation with a PCR sDPT gene-specific probe gave no signal in wild type plants and showed that most transformed plants had one to four sDPT gene copies of varying length, suggesting a complex T-DNA insertion pattern from putatively independent integration events. Additional faint bands probably resulting from partially digested DNA were also visible in some samples (Fig. 3).
To check if the inserted sDPT gene was transcribed, RT-PCR was conducted with total RNA isolated from one wild type and 11 transgenic plants of independent origin. Using actin-mRNA as an internal control, sDPT transcripts were not detected in wild type leaves whereas the expected 570 bp signal was present in all transgenic plants analysed, indicating that the synthetic gene was transcribed (Fig. 4).
sDPT polypeptide content in tomato
Enzyme immunoassays were used to estimate the sDPT polypeptide content in leaf and fruit tissues. In each essay a tomato leaf or fruit extracts from untrasformed plant was included. The reading at 450 nm of the wild type was subtracted of the reading of the transgenic plants. The amounts of diphtheria, pertussis and tetanus exotoxin antigens relative to the total soluble leaf and fruit protein in T0 plants were calculated in replicate assays on each tested plant.
For diphtheria antigens the range of sDPT protein content was 1.9–5.8 μg g−1 fresh weight in leaves and 1–2.1 μg g−1 fresh weight in fruits. For tetanus antigens the range was 4.5–17 μg g−1 fresh weight in leaves and 2.25–6.8 μg g−1 fresh weight in fruits. The pertussis antigen content range in transformed plants was 6.6 × 10−4–1,530 × 10−4 μg g−1 fresh weight in leaves and 3.4 × 10−4–291 × 10−4 μg g−1 fresh weight in fruit. Standard curves for diphtheria and tetanus toxoids were produced with the DPT vaccine (Secretaría de Salud, Mexico, clave 3805); the commercial Quadracel™ vaccine (Pasteur Mérieux Connaught, Rhone-Poulenc Group) was used for pertussis toxoid quantification (Fig. 5).
Discussion
Development of multicomponent vaccines is a priority of current vaccine research. One of the most successful combined vaccines in use is the DPT vaccine, but concerns about the safety of the pertussis arm have led to decreased acceptance of the vaccine and the development of new, safer, effective DPT vaccines. Unfortunately, the cost of producing these new vaccines is significantly higher than that of the old ones.
Previous reports have shown that plants can express antigens at high levels in their native form (Fischer and Emans 2000). The antigens remain encapsulated in plant tissues and therefore subjected to a less harsh environment in the digestive system. With this approach a plant based multicomponent vaccine was developed by Yu and Langridge (2001), who fused the cholera toxin A2 and B subunits to the rotavirus enterotoxin to protect against enteric diseases. Recently, the fragment C of tetanus toxin expressed in tobacco chloroplasts has been shown to protected mice against lethal tetanus toxin challenge (Tregoning et al. 2005).
In this work we achieved transformation of tomatoes with sDPT, a multiepitope synthetic gene encoding two immunodominant protective epitopes of each of the diphteria, pertussis and tetanus exotoxins, which were expressed in a polypeptide antigenically active in vitro. The sDPT codons were plant-optimised as this approach has been shown to enhance the expression of bacterial genes in plants (Gribskov et al. 1984; Perlak et al. 1991; Adang et al. 1993; Horvath et al. 2000).
From the Southern blot assay we concluded that eight independent transgenic tomato plants were identified with the sDPT gene. Southern blot analysis showed that most of the transgenic events resulted in at least one copy of the transgene cassette. All the transgenic plants confirmed by Southern blotting were also shown to express the expected 570-bp transcript by RT-PCR.
The genomic DNA and cDNA showed in Figs. 3 and 4 (lanes 1–11) correspond to the transgenic plants T32, T25, T21, T18, T17, T15, T14, T6, T4, T2 and T1, respectively, showed in Fig. 5. There is no correlation with the copy number shown in the Southern analysis and the protein detection by ELISA. Line T17 with only one copy (lane 5 in Fig. 3) was the plant with highest protein content both for diphtheria and tetanus in leaf. Also in the RT-PCR analysis although we did not quantified the expression level normalising the amplicons vs the actin, T17 (lane 5 in Fig. 4) shows lower transcript levels than T18 (lane 4 in Fig. 4). We think that different posttranscriptional or posttranslational regulation effects due to different insertion sites could be the reason of these differences. An additional Western blot analysis may help us to better understand the possible modifications that the polypeptide may undergo. What is interesting is that in all leaf and fruit samples the pertussis was lower that the other two antigenic peptides in spite of being in the same molecule, it might be the SEKDEL sequence close to the pertussis can be influencing in the proper exposition for the antibody recognition, further work should be done to improve the design on the pertussis epitopes. It was not possible to explain why lines T14 and T17 which showed high protein values for diphtheria and tetanus in leaf then showed low values for pertussis. It might be a different degradation profiles in the C-terminal peptide for these lines. In the other hand we observed that the integration pattern of the transgene showed similar bands in lanes 6–11 (Fig. 3) which could indicate an especial array conserved in different transformation events. T-DNA is not always integrated in a complete way and some times even part of the binary vector is integrated (Herman et al. 1990; Yin and Wang 2000).
There are no reports of pertussis and diphtheria toxin expression in plants and there is only one report of tetanus toxin fragment C expression in chloroplast transformed plants reaching 10–25% of the total soluble protein (TSP) content (Tregoning et al. 2005). Although such levels are very high they are not surprising, since the expression attained through chloroplast transformation is 10–25 times higher than through nuclear transformation (Daniell et al. 2005). The sDPT polypeptide expressed in transgenic tomatoes contains only two epitopes from each of the three exotoxins, whose content in vaccine toxoids is indirectly determined with the polyclonal antibodies of the antisera prepared to titrate each toxoid. Assuming that the sDPT polypeptide contains all three exotoxin epitopes, the polyclonal antibodies used to titrate it would indicate that its content in our transgenic tomato lines was >0.01% TSP. Although this value is much lower than that reported for chloroplast transformants, and although we did not express the non-optimised gene for comparison, we believe that it still is around five times higher than that obtained by others when they tried to express native bacterial genes (Haq et al. 1995), supporting the higher efficiency of synthetic gene expression.
We demonstrated sDPT polypeptide expression through the antigenic activity of tissue extracts with specific antibodies to the diphtheria, pertussis and tetanus exotoxins. To our knowledge this is the first report of a successful recombinant fusion protein that could work as a multicomponent DPT subunit vaccine, whose oral immunogenicity with T0 plants is currently being tested.
Abbreviations
- DPT:
-
Diphtheria–pertussis–tetanus
- RT-PCR:
-
Reverse-transcriptase polymerase chain reaction
- MS:
-
Murashige and Skoog medium
- IAA:
-
Indole-3-acetic acid
- NAA:
-
Naphthalene acetic acid
- BAP:
-
6-Benzylaminopurine
References
Adang MJ, Brody MS, Cardineau G (1993) The reconstruction and expression of a Bacillus thuringiensis cryIIA gene in protoplasts and potato plants. Plant Mol Biol 21:1131–1145
Baraff LJ, Ablon WJ, Weiss RC (1983) Possible temporal association between diphtheria–tetanus toxoid-pertussis-vaccination and sudden infant death syndrome. Ped Infect Dis 2:7–11
Boucher P, Sato H, Sato Y, Locht C (1994) Neutralizing antibodies and immunoprotection against pertussis and tetanus obtained by use of a recombinant pertussis toxin-tetanus toxin fusion protein. Infect Immun 62: 449–456
Bradford MM (1979) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Cavanaugh NP, Brett EM, Marshall WC, Wilson J (1981) The possible adjuvant role of Bordetella pertussis and pertussis vaccine in causing severe encephalopathic illness: a presentation of three case histories. Neuropediatrics 12:374–381
Cortina C, Culiañez-Macià FA (2004) Tomato transformation and transgenic plant production. Plant Cell Tissue Organ Cult 76:269–275
Daniell H, Streatfield SJ, Wycoff K (2001) Medical molecular farming: production of antibodies, biopharmaceuticals and edible vaccines in plants. Trends Plant Sci 6:219–226
Daniell H, Chebolu S, Kumar S, Singleton M, Falconer R (2005) Chloroplast-derived vaccine antigens and other therapeutic proteins. Vaccine 23:1779–1783
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: version I. Plant Mol Biol Rep 1:19–21
Fischer R, Emans N (2000) Molecular farming of pharmaceutical proteins. Transgenic Res 9:279–299
Gerathy KC (1984) DPT Immunization and SIDS. J Pediatr 105:169–170
Gribskov M, Devereux J, Burgess RR (1984) The codon preference plot: graphic analysis of protein coding sequences and prediction of gene expression. Nucl Acids Res 12:539–549
Haq TA, Mason HS, Clements JD, Arntzen CJ (1995) Oral Immunization with a recombinant bacterial antigen produced in transgenic plants. Science 268:714–716
Herman I, Jacobs A, Van Montagu M, Depicker A (1990) Plant chromosome/marker gene fusion assay for study of normal and truncated T-DNA integration events. Mol Gen Genet 224:248–256
Hinman AR, Koplan J (1984) Pertussis and pertussis vaccine: reanalysis of benefits, risks, and costs. JAMA 251:3109–3113
Horn ME, Woodard SL, Howard JA (2004) Plant molecular farming: systems and products. Plant Cell Rep 22:711–720
Horvath H, Huang J, Wong O, Kohl E, Okita T, Kannangara CG (2000) The production of recombinant protein in transgenic barley grains. Proc Natl Acad Sci USA 97:1914–1919
Jani D, Singh NK, Bhattacharya S, Meena LS, Singh Y, Upadhyay SN, Sharma AK, Tyagi AK (2004) Studies on the immunogenic potential of plant-expressed cholera toxin B subunit. Plant Cell Rep 22:471–477
Kim TG, Langridge WH (2003) Assembly of cholera toxin B subunit full-length rotavirus NSP4 fusion protein oligomers in transgenic potato. Plant Cell Rep 21:884–890
Kim TG, Gruber A, Langridge WH (2004) HIV-1 gp120 V3 cholera toxin B subunit fusion gene expression in transgenic potato. Protein Expr Purif 37:196–202
Koplan JP, Schoenbaum SC, Weinstein MC, Fraser DW (1979) Pertussis vaccine—an analysis of benefits, risks and costs. New Engl J Med 301:906–911
Lee SF, Halperin SA, Knight JB, Tait A (2002) Purification and immunogenicity of a recombinant Bordetella pertussis S1S3FHA fusion protein expressed by Streptococcus gordonii. Appl Env Microbiol 68:4253–4258
Lobeck K, Drevet P, Leonetti M, Fromen-Romano C, Ducancel F, Lajeunesse C, Lemaire C, Menez A (1998) Towards a recombinant vaccine against diphtheria toxin. Infect Immun 66:418–423
McGarvey PB, Hammond J, Dienelt MM, Hooper DC, Fu ZF, Dietzschold B, Koprowski H, Michaels FH (1995) Expression of the rabies virus glycoprotein in transgenic tomatoes. Biotechnology 13:1484–1487
Meszaros JR, Asch DA, Baron J, Hershey JC, Kunreuther H, Schwartz-Buzaglo J (1996) Cognitive processes and the decisions of some parents to forego pertussis vaccination for their children. J Clin Epidemiol 49:697–703
Moffat AS (1995) Exploring transgenic plants as a new vaccine source. Science 268:658–660
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–479
Perlak FJ, Fuchs RL, Dean DA, McPherson SL, Fischhoff DA (1991) Modification of the coding sequence enhances plants expression of insect control protein genes. Proc Natl Acad Sci USA 88:3324–3328
Ribas AV, Ho PL, Tanizaki MM, Raw I, Nascimento AL (2000) High-level expression of tetanus toxin fragment C-thioredoxin fusion protein in Escherichia coli. Biotechnol Appl Biochem 31:91–94
Rigano MM, Walmsley AM (2005) Expression systems and developments in plant-made vaccines. Immunol Cell Biol 83:271–277
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Tacket CO, Mason HS, Losonsky G, Clements JD, Wasserman SS, Levine MM, Arntzen CJ (1998) Immunogenicity in humans of a recombinant bacterial-antigen delivered in transgenic potato. Nat Med 4:607–609
Thanavala Y, Yang YF, Lyons P, Mason HS, Arntzen CJ (1995) Immunogenicity of transgenic plant-derived hepatitis B surface antigen. Proc Natl Acad Sci USA 92:3358–3361
Tortorella D, Sesardic D, Dawes CS, London E (1995) Immunochemical analysis of the structure of diphtheria toxin shows all three domains undergo structural changes at low pH. J Biol Chem 17:27439–27445
Tregoning JS, Clare S, Bowe F, Edwards L, Fairweather N, Qazi O, Nixon PJ, Maliga P, Dougan G, Hussell T (2005) Protection against tetanus toxin using a plant-based vaccine. Eur J Immunol 35: 1320–1326
Yin Z, Wang GL (2000) Evidence of multiple complex patterns of T-DNA integration into the rice genome. Theor Appl Genet 100:461–470
Yu J, Langridge WH (2001) A plant-based multicomponent vaccine protects mice from enteric diseases. Nat Biotechnol 19:548–552
Acknowledgments
We thank Dr. Song Lee (Dalhousie University) who generously provided the antibodies to pertussis toxin and the Quadracel™ vaccine. This work was partially supported by grant 37048-B from CONACYT Mexico and scholarships 172307 and 165946 for the graduate studies of Ruth Soria-Guerra and Sergio Rosales-Mendoza.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Lakshmanan.
Rights and permissions
About this article
Cite this article
Soria-Guerra, R.E., Rosales-Mendoza, S., Márquez-Mercado, C. et al. Transgenic tomatoes express an antigenic polypeptide containing epitopes of the diphtheria, pertussis and tetanus exotoxins, encoded by a synthetic gene. Plant Cell Rep 26, 961–968 (2007). https://doi.org/10.1007/s00299-007-0306-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-007-0306-y