Abstract
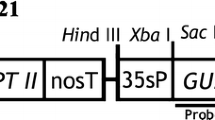
A system for the production of transgenic plants was developed for the Oriental hybrid lily, Lilium cv. Acapulco, by Agrobacterium-mediated genetic transformation. Filament-derived calli were co-cultivated with A. tumefaciens strain EHA101/pIG121Hm, which harbored a binary vector carrying the neomycin phosphotransferase II, hygromycin phosphotransferase, and intron-containing β-glucuronidase genes in the T-DNA region. Six hygromycin-resistant (Hygr) culture lines were obtained from 200 calli by scratching them with sandpaper prior to inoculation and using NH4NO3-free medium for co-cultivation and a hygromycin-containing regeneration medium for selection. Hygr culture lines regenerated shoots, which developed into plantlets following transfer to a plant growth regulator-free medium. All of these plantlets were verified to be transgenic by GUS histochemical assay and inverse PCR analysis.


Similar content being viewed by others
Abbreviations
- AS :
-
Acetosyringone (3,5-dimethoxy-4-hydroxy-acetophenone)
- BA :
-
Benzyladenine
- CaMV :
-
Cauliflower mosaic virus
- GUS :
-
β-Glucuronidase
- HPT :
-
Hygromycin phosphotransferase
- Hyg r :
-
Hygromycin-resistant
- NOS :
-
Nopaline synthase
- NPTII :
-
Neomycin phosphotransferase II
- PGR :
-
Plant growth regulator
- PIC :
-
Picloram (4-amino-3,5,6-trichloropicolinic acid)
References
Bennett MD, Smith JB (1991) Nuclear DNA amounts in angiosperms. Philos Trans R Soc London Ser B 334:309–345
Bidney D, Scelonge C, Martich J, Burrus M, Sims L, Huffman G (1992) Microprojectile bombardment of plant tissues increases transformation frequency by Agrobacterium tumefaciens. Plant Mol Biol 18:301–313
Brasileiro ACM, Aragäo FJL, Rossi S, Dusi DMA, Gomes Barros LM, Rech EL (1996) Susceptibility of common and tepary beans to Agrobacterium spp. strains and improvement of Agrobacterium-mediated transformation using microprojectile bombardment. J Am Soc Hortic Sci 121:810–815
Cheng YH, Yang JS, Yeh SD (1996) Efficient transformation of papaya by coat protein gene of papaya ringspot virus mediated by Agrobacterium following liquid-phase wounding of embryogenic tissues with carborundum. Plant Cell Rep 16:127–132
Cordero de Mesa M, Jiménez-Bermúdez S, Pliego-Alfaro F, Quesada MA, Mercado JA (2000) Agrobacterium cells as microprojectile coating: a novel approach to enhance stable transformation rates in strawberry. Aust J Plant Physiol 27:1093–1100
Does MP, Dekker BMM, de Groot MJA, Offringa R (1991) A quick method to estimate the T-DNA copy number in transgenic plants at an early stage after transformation, using inverse PCR. Plant Mol Biol 17:151–153
Eady CC, Weld RJ, Lister CE (2000) Agrobacterium tumefaciens-mediated transformation and transgenic-plant regeneration of onion (Allium cepa L.). Plant Cell Rep 19:376–381
Fladung M (1999) Gene stability in transgenic aspen (Populus). I. Flanking DNA sequences and T-DNA structure. Mol Gen Genet 260:574–581
Gheysen G, Van Montagu M, Zambryski P (1987) Integration of Agrobacterium tumefaciens T-DNA involves rearrangements of target DNA sequences. Proc Natl Sci USA 84:6169–6173
Grayburn WS, Vick BA (1995) Transformation of sunflower (Helianthus annuus L.) following wounding with glass beads. Plant Cell Rep 14:285–289
Han DS, Niimi Y, Nakano M (1997) Regeneration of haploid plant from anther culture of the Asiatic hybrid lily 'Connecticut King'. Plant Cell Tissue Organ Cult 47:153–158
Hiei Y, Komari T, Ishida Y, Saito H (2000) Development of Agrobacterium-mediated transformation method for monocotyledonous plants. Breed Res 2:205–213
Iglesias VA, Moscone EA, Papp I, Neuhuber F, Michalowski S, Phelan T, Spiker S, Matzke M, Matzke AJM (1997) Molecular and cytogenetic analyses of stably and unstably expressed transgene loci in tobacco. Plant Cell 9:1251–1264
Jefferson RA (1987) Assaying chimeric genes in plant: the GUS gene fusion system. Plant Mol Biol Rep 5:387–405
Kisaka H, Kameya T (1998) Fertile transgenic asparagus plants produced by Agrobacterium-mediated transformation. Plant Biotechnol 15:177–181
Knittel N, Gruber V, Hahne G, Lénée P (1994) Transformation of sunflower (Helianthus annuus L.): a reliable protocol. Plant Cell Rep 14:81–86
Koncz C, Martini N, Mayerhofer R, Koncz-Kálmán Z, Köber H, Redei GP, Schell J (1989) High-frequency T-DNA-mediated gene tagging in plants. Proc Natl Acad Sci USA 86:8467–8471
Kondo T, Hasegawa H, Suzuki M (2000) Transformation and regeneration of garlic (Allium sativum L.) by Agrobacterium-mediated gene transfer. Plant Cell Rep 19:989–993
Langeveld SA, Gerrits MM, Derks AFLM, Boonekamp PM, Bol JF (1995) Transformation of lily by Agrobacterium. Euphytica 85:97–100
Lichtenstein CP, Draper J (1985) Genetic engineering of plants. In: Glover DM (ed) DNA cloning: a practical approach, vol 2. IRL Press, Washington D.C., p 78
Mathur J, Szabados L, Schaefer S, Grunenberg B, Lossow A, Esther J-S, Schell J, Koncz C, Koncz-Kálmán Z (1998) Gene identification with sequenced T-DNA tags generated by transformation of Arabidopsis cell suspension. Plant J 13:707–716
Miyoshi H, Usami T, Tanaka I (1995) High level of GUS gene expression driven by pollen-specific promoters in electroporated lily pollen protoplasts. Sex Plant Reprod 8:205–209
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nishihara M, Ito M, Tanaka I, Kyo M, Ono K, Irifune K, Morikawa M (1993) Expression of the ß-glucuronidase gene in pollen of lily (Lilium longiflorum), tobacco (Nicotiana tabacum), Nicotiana rustica, and peony (Paeonia lactiflora) by particle bombardment. Plant Physiol 102:357–361
Ochman H, Gerber AS, Hartl DL (1988) Genetic applications of an inverse polymerase chain reaction. Genetics 120:621–623
Ohta S, Mita S, Hattori T, Nakamura K (1990) Construction and expression in tobacco of a β-glucuronidase (GUS) reporter gene containing an intron within the coding sequence. Plant Cell Physiol 31:805–813
Potrykus I (1991) Gene transfer to plants: assessment of published approaches and results. Annu Rev Plant Physiol Plant Mol Biol 42:205–225
Robinson K, Firoozababy E (1993) Transformation of floriculture crops. Sci Hortic 55:83–99
Sanford JC, Smith FD, Russell JA (1993) Optimizing the biolistic process for different biological applications. Methods Enzymol 217:483–510
Suzuki S, Nakano M (2002) Agrobacterium-mediated production of transgenic plants Muscari armeniacum Leichtl. ex Bak. Plant Cell Rep 20:835–841
Suzuki S, Niimi Y, Sakakibara T, Hosokawa K, Yamamura S, Nakano M (1998) Effects of several antibiotics and bialaphos on the growth and organ formation of Lilium formosanum calli and transient expression of the gusA gene after co-cultivation with Agrobacterium tumefaciens. Plant Biotechnol 15:213–216
Suzuki S, Supaibulwatana K, Mii M, Nakano M (2001) Production of transgenic plants of Liliaceous ornamental plant Agapanthus praecox ssp. orientalis (Leighton) Leighton via Agrobacterium-mediated transformation of embryogenic calli. Plant Sci 161:89–97
Triglia T, Peterson MG, Kemp DJ (1988) A procedure for in vitro amplification of DNA segments that lie outside the boundaries of known sequences. Nucleic Acids Res 16:8186
Tsuchiya T, Takumi S, Shimada T (1996) Transient expression of a reporter gene in bulbscales and immature embryos of three Lilium species is affected by 5′ upstream sequences and culture conditions. Physiol Plant 98:699–704
Watad AA, Yun D-J, Matsumoto T, Niu X, Wu Y, Kononowicz AK, Bressan RA, Hasegawa PM (1998) Microprojectile bombardment-mediated transformation of Lilium longiflorum. Plant Cell Rep 17:262–267
Wilmink, A, van de Ven BCE, Dons JJM (1995) Activity of constitutive promoters in various species from the Liliaceae. Plant Mol Biol 28:949–955
Zheng S-J, Henken B, Sofiari E, Jacobsen E, Krens FA, Kik C (2001) Molecular characterization of transgenic shallots (Allium cepa L.) by adaptor ligation PCR (AL-PCR) and sequencing of genomic DNA flanking T-DNA borders. Transgen Res 10:237–245
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Ebinuma
Rights and permissions
About this article
Cite this article
Hoshi, Y., Kondo, M., Mori, S. et al. Production of transgenic lily plants by Agrobacterium-mediated transformation. Plant Cell Rep 22, 359–364 (2004). https://doi.org/10.1007/s00299-003-0700-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-003-0700-z