Abstract
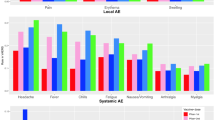
This study aimed to investigate the reporting rates of arthritis and arthralgia following the administration of four vaccines against SARS-CoV-2: Pfizer-BioNTech (Tozinameran), Moderna (CX-024414), AstraZeneca (Chadox1 NCOV-19), and Janssen (AD26.COV2.S) in 2021. We used data from the EudraVigilance database, specifically analyzing spontaneous reports of suspected adverse reactions (ADRs) from the European Union (EU)/European Economic Area (EEA) region. Age-group-specific reporting rates were calculated by dividing the number of arthralgia and arthritis reports per 1,000,000 vaccine doses administered per age group. Reporting rates were compared using a rate ratio among the four vaccines, using the AstraZeneca vaccine as a comparator. The AstraZeneca vaccine was associated with the highest rate of arthralgia across all age groups. Arthritis reporting rates were significantly lower, with the AstraZeneca vaccine having the highest rates in most age groups, except the 60–69 and 80+ groups, where the Janssen and Pfizer-BioNTech vaccines demonstrated higher reporting rates, respectively. The distribution of arthritis rates did not follow the arthralgia pattern, being higher in the 50–79 age group. This study is the first spontaneous reporting system analysis of arthritis reporting rates post-SARS-CoV-2 vaccination at a European level, revealing a higher reporting of suspected musculoskeletal adverse reactions after AstraZeneca vaccination. The findings underscore the need to consider commonly reported events like arthralgia in risk–benefit assessments prior to vaccination against SARS-CoV-2. Given the high prevalence of rheumatic and musculoskeletal diseases and vaccine hesitancy in this population, our results could influence vaccine choice and acceptance.



Similar content being viewed by others
Data availability
Data are available upon reasonable request from the authors.
References
Guimarães LE, Baker B, Perricone C, Shoenfeld Y (2015) Vaccines, adjuvants and autoimmunity. Pharmacol Res 100:190–209. https://doi.org/10.1016/j.phrs.2015.08.003
Cross JW, Joy M, McGee C, Akinyemi O, Gatenby P, de Lusignan S (2020) Adverse events of interest vary by influenza vaccine type and brand: sentinel network study of eight seasons (2010–2018). Vaccine 38:3869–3880. https://doi.org/10.1016/j.vaccine.2020.03.034
Centers for Disease Control and Prevention (2023) Seasonal Flu Vaccines. Centers for Disease Control and Prevention. https://www.cdc.gov/flu/prevent/flushot.htm. Accessed 31 May 2023
European Centre for Disease Prevention and Control (2023) COVID-19 Vaccine Tracker. European Centre for Disease Prevention and Control – An agency of the European Union. https://www.ecdc.europa.eu/en/publications-data/covid-19-vaccine-tracker. Accessed 8 Jan 2023
Cari L, Fiore P, Naghavi Alhosseini M, Sava G, Nocentini G (2021) Blood clots and bleeding events following BNT162b2 and ChAdOx1 nCoV-19 vaccine: an analysis of European data. J Autoimmun 122:102685. https://doi.org/10.1016/j.jaut.2021.102685
Driedger MS, Capurro G, Tustin J, Jardine CG (2023) “I won’t be a guineapig”: Rethinking public health communication and vaccine hesitancy in the context of COVID-19. Vaccine 41:1–4. https://doi.org/10.1016/j.vaccine.2022.11.056
Medical Dictionary for Regulatory Activities (2021) MedDRA Version 24.1 September 2021. Medical Dictionary for Regulatory Activities. https://www.meddra.org/how-to-use/support-documentation/english/welcome. Accessed 19 March 2023
European Centre for Disease Prevention and Control (2022). Data on COVID-19 Vaccination in the EU/EEA. European Centre for Disease Prevention and Control – An agency of the European Union. https://www.ecdc.europa.eu/en/publications-data/data-covid-19-vaccination-eu-eea. Accessed 15 Aug 2022
Klok FA, Pai M, Huisman MV, Makris M (2022) Vaccine-induced immune thrombotic thrombocytopenia. Lancet Haematol 9:73–80. https://doi.org/10.1016/S2352-3026(21)00306-9
Medicines and Healthcare Products Regulatory Agency (2023). Coronavirus Vaccines – Summary of Yellow Card Reporting - Data included 9/12/2020 to 20/4/2022. Medicines and Healthcare Products Regulatory Agency. https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting. Accessed 5 Apr 2023
Montano D (2022) Frequency and associations of adverse reactions of COVID-19 vaccines reported to pharmacovigilance systems in the European union and the United States. Front Public Health 9:756633. https://doi.org/10.3389/fpubh.2021.756633
Prontskus V, Fresse A, Yelehe-Okouma M, Facile A, Pietri T, Simon C, Le Souder C, Beurrier M, Gillet P, French network of Regional Pharmacovigilance Centres (2023) COVID-19 vaccination and the incidence of De Novo or recurrent rheumatoid arthritis: a French and international (VigiBase) signal detection study. Clin Pharmacol Ther 113:1107–1116. https://doi.org/10.1002/cpt.2866
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Ménard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovský J, Wolfe F, Hawker G (2010) 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 62:2569–2581. https://doi.org/10.1002/art.27584
Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, Sudre CH, Nguyen LH, Drew DA, Merino J, Hu C, Selvachandran S, Antonelli M, Murray B, Canas LS, Molteni E, Graham MS, Modat M, Joshi AD, Mangino M, Hammers A, Goodman AL, Chan AT, Wolf J, Steves CJ, Valdes AM, Ourselin S, Spector TD (2021) Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis 21:939–949. https://doi.org/10.1016/S1473-3099(21)00224-3
Anastassopoulou C, Hatziantoniou S, Vlachopoulos C, Spanakis N, Tsioufis C, Tsakris A, Lazaros G (2022) Temporal relationship of myocarditis and pericarditis following COVID-19 vaccination: a pragmatic approach. Int J Cardiol 358:136–139. https://doi.org/10.1016/j.ijcard.2022.04.024
MacDonald NE, SAGE Working Group on Vaccine Hesitancy (2015) Vaccine hesitancy: definition, scope and determinants. Vaccine 33:4161–4164. https://doi.org/10.1016/j.vaccine.2015.04.036
Priori R, Pellegrino G, Colafrancesco S, Alessandri C, Ceccarelli F, Di Franco M, Riccieri V, Scrivo R, Sili Scavalli A, Spinelli FR, Conti F (2021) SARS-CoV-2 vaccine hesitancy among patients with rheumatic and musculoskeletal diseases: a message for rheumatologists. Ann Rheum Dis 80:953–954. https://doi.org/10.1136/annrheumdis-2021-220059
Lazarus JV, Wyka K, White TM, Picchio CA, Rabin K, Ratzan SC, Parsons Leigh J, Hu J, El-Mohandes A (2022) Revisiting COVID-19 vaccine hesitancy around the world using data from 23 countries in 2021. Nat Commun 13:3801. https://doi.org/10.1038/s41467-022-31441-x
Gaur P, Agrawat H, Shukla A (2021) COVID-19 vaccine hesitancy in patients with systemic autoimmune rheumatic disease: an interview-based survey. Rheumatol Int 41:1601–1605. https://doi.org/10.1007/s00296-021-04938-9
Yurttas B, Poyraz BC, Sut N, Ozdede A, Oztas M, Uğurlu S, Tabak F, Hamuryudan V, Seyahi E (2021) Willingness to get the COVID-19 vaccine among patients with rheumatic diseases, healthcare workers and general population in Turkey: a web-based survey. Rheumatol Int 41:1105–1114. https://doi.org/10.1007/s00296-021-04841-3
Sen P, Roh N, Houshmand N, Moghadam Kia S, Joshi M, Saha S, Jagtap K, Agarwal V, Nune A, Nikiphorou E, Tan AL, Shinjo SK, Ziade N, Velikova T, Milchert M, Parodis I, Gracia-Ramos AE, Cavagna L, Kuwana M, Knitza J, Makol A, Patel A, Pauling JD, Wincup C, Barman B, Zamora Tehozol EA, Rojas Serrano J, García-De La Torre I, Colunga-Pedraza IJ, Merayo-Chalico J, Chibuzo OC, Katchamart W, Akawatcharangura Goo P, Shumnalieva R, Chen YM, Hoff LS, El Kibbi L, Halabi H, Vaidya B, Sazliyana Shaharir S, Hasan ATMT, Dey D, Gutiérrez CET, Caballero-Uribe CV, Lilleker JB, Salim B, Gheita T, Chatterjee T, Distler O, Saavedra MA, Day J, Chinoy H, COVAD Study Group, Agarwal V, Aggarwal R, Gupta L (2023) Vaccine hesitancy decreases in rheumatic diseases, long-term concerns remain in myositis: a comparative analysis of the COVAD surveys. Rheumatology (Oxford) 62:3291–3301. https://doi.org/10.1093/rheumatology/kead057
Gastelum-Strozzi A, Flores-Alvarado DE, Pascual-Ramos V, Álvarez-Hernández E, Pacheco-Tena CF, Guaracha-Basáñez GA, García CG, González-Chávez SA, Moctezuma-Ríos JF, Manrique de Lara A, Esquivel-Valerio JA, Contreras-Yáñez I, Galarza-Delgado DÁ, Vázquez-Mellado J, Peláez-Ballestas I, Reyes-Cordero GC (2023) The COVID-19 epidemic curve and vaccine acceptance among patients with rheumatic diseases: an ecological study. Rheumatol Int 43:1253–1264. https://doi.org/10.1007/s00296-023-05334-1
Paik JJ, Sparks JA, Kim AHJ (2022) Immunogenicity, breakthrough infection, and underlying disease flare after SARS-CoV-2 vaccination among individuals with systemic autoimmune rheumatic diseases. Curr Opin Pharmacol 65:102243. https://doi.org/10.1016/j.coph.2022.102243
Edwards KM, Orenstein WA (2023) COVID19: Vaccines. Uptodate. https://www.uptodate.com/contents/covid-19-vaccines. Accessed 31 May 2023
Shiravi AA, Ardekani A, Sheikhbahaei E, Heshmat-Ghahdarijani K (2022) Cardiovascular complications of SARS-CoV-2 vaccines: an overview. Cardiol Ther 11:13–21. https://doi.org/10.1007/s40119-021-00248-0
Marcec R, Likic R (2022) Using Twitter for sentiment analysis towards AstraZeneca/Oxford, Pfizer/BioNTech and Moderna COVID-19 vaccines. Postgrad Med J 98:544–550. https://doi.org/10.1136/postgradmedj-2021-140685
Biswas MR, Alzubaidi MS, Shah U, Abd-Alrazaq AA, Shah Z (2021) A scoping review to find out worldwide COVID-19 vaccine hesitancy and its underlying determinants. Vaccines (Basel) 9:1243. https://doi.org/10.3390/vaccines9111243
Acknowledgements
The authors are grateful to Mr. Rodrigo Postigo from EudraVigilance for providing insightful comments on the manuscript prior to its initial submission. Furthermore, the authors are grateful to EudraVigilance for providing access to the requested data.
Funding
No funding was provided for this study.
Author information
Authors and Affiliations
Contributions
EPP, RM, MZ, TK, and IP: wrote the manuscript and performed data analysis, while BA and RL: performed critical revision and coordination for the study. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None to declare.
Ethical approval
Thical Committee of the School of Medicine, University of Zagreb, October 27, 2021, number 380-59-10106-21-111/230, class 641-01/21-02/01.
Patient and public involvement
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Primorac Padjen, E., Marcec, R., Zidar, M. et al. Comparison of reporting rates of arthritis and arthralgia following AstraZeneca, Pfizer-BioNTech, Moderna, and Janssen vaccine administration against SARS-CoV-2 in 2021: analysis of European pharmacovigilance large-scale data. Rheumatol Int 44, 273–281 (2024). https://doi.org/10.1007/s00296-023-05512-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-023-05512-1