Abstract
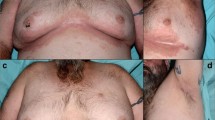
Alopecia areata (AA) is a common non-scaring hair loss associated with many inflammatory and autoimmune disorders. Anti-tumor necrosis factor alpha (TNFα) therapy is used to treat many chronic inflammatory disorders and has been proven to be effective and relatively safe. However, several immune-mediated skin reactions have been described with the use of TNFα inhibitors, among them AA. In this report, we describe two patients, a 32-year-old woman with ankylosing spondylitis and a 48-year-old man with rheumatoid arthritis who were both treated with SB4 (Benepali®), an etanercept biosimilar, and developed AA, 6 and 12 months respectively after the initiation of TNFα blocker biosimilar. These, are the first two cases of AA development during TNFα inhibitors biosimilar. Thus, physicians when dealing with patients treated with these agents, should be aware of possible immune skin reactions, among them AA. To this end, a close follow-up and monitoring is mandatory.


Similar content being viewed by others
Availability of data and material
Not applicable.
References
Fukuyama M, Ito T, Ohyama M (2022) Alopecia areata: current understanding of the pathophysiology and update on therapeutic approaches, featuring the Japanese Dermatological Association guidelines. J Dermatol 49:19–36
Sfikakis PP, Bournia VK, Sidiropoulos P et al (2017) Biologic treatment for rheumatic disease: real-world big data analysis from the Greek country-wide prescription database. Clin Exp Rheumatol 35:579–585
Flouri I, Markatseli TE, Voulgari PV et al (2014) Comparative effectiveness and survival of infliximab, adalimumab, and etanercept for rheumatoid arthritis patients in the Hellenic Registry of Biologics: low rates of remission and 5-year drug survival. Semin Arthritis Rheum 43:447–457
Her M, Kavanaugh A (2016) Alterations in immune function with biologic therapies for autoimmune disease. J Allergy Clin Immunol 137:19–27
Boyman O, Comte D, Spertini F (2014) Adverse reactions to biologic agents and their medical management. Nat Rev Rheumatol 10:612–627
Drosos AA, Pelechas E, Kaltsonoudis E et al (2021) Biologic therapies and autoimmune phenomena. Mediterr J Rheumatol 32:96–103
Ramos-Casals M, Brito-Zerón P, Muñoz S et al (2007) Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore) 86:242–251
Exarchou SA, Voulgari PV, Markatseli TE et al (2009) Immune-mediated skin lesions in patients treated with anti-tumour necrosis factor alpha inhibitors. Scand J Rheumatol 38:328–331
Sfikakis PP, Iliopoulos A, Elezoglou A et al (2005) Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum 52:2513–2518
Voulgari PV, Markatseli TE, Exarchou SA et al (2008) Granuloma annulare induced by anti-tumour necrosis factor therapy. Ann Rheum Dis 67:567–570
Masukawa E, Matsushima Y, Habe K et al (2021) Two cases of cutaneous adverse effects induced by tumor necrosis factor-alpha inhibitors. Case Rep Dermatol 13:238–243
Youssef R, Saad S, Hendrick S (2020) Alopecia universalis and onychodystrophy during treatment with adalimumab. Proc (Bayl Univ Med Cent) 33:596–597
Ostojic P, Pavlov-Dolijanovic S (2018) Alopecia universalis in a patient with rheumatoid arthritis developed during treatment with adalimumab. Z Rheumatol 77:412–415
Melé-Ninot G, Expósito-Serrano V, Quintana Codina M et al (2017) Adalimumab-related alopecia in a patient affected by psoriasis. Dermatol Online J. https://doi.org/10.5070/D3237035733
Craddock LN, Cooley DM, Endo JO et al (2017) TNF inhibitor induced alopecia: an unusual form of psoriasiform alopecia that breaks the Renbök mold. Dermatol Online J. https://doi.org/10.5070/D3233034290
Toda-Brito H, Lopes L, Soares-Almeida L et al (2015) Adalimumab-induced psoriatic alopecia/alopecia areata-like reaction in a patient with Crohn’s disease. Dermatol Online J. https://doi.org/10.5070/D32111029286
Ribeiro LB, Rego JC, Estrada BD et al (2015) Alopecia secondary to anti-tumor necrosis factor-alpha therapy. An Bras Dermatol 90:232–235
Beccastrini E, Squatrito D, Emmi G et al (2010) Alopecia areata universalis during off-label treatment with infliximab in a patient with Behçet disease. Dermatol Online J 16:15
Hernández MV, Nogués S, Ruiz-Esquide V et al (2009) Development of alopecia areata after biological therapy with TNF-alpha Blockers: description of a case and review of the literature. Clin Exp Rheumatol 27:892–893
Kirshen C, Kanigsberg N (2009) Alopecia areata following adalimumab. J Cutan Med Surg 13:48–50
Pan Y, Rao NA (2009) Alopecia areata during etanercept therapy. Ocul Immunol Inflamm 17:127–129
Chaves Y, Duarte G, Ben-Said B et al (2008) Alopecia areata universalis during treatment of rheumatoid arthritis with anti-TNF-alpha antibody (adalimumab). Dermatology 217:380
Garcia Bartels N, Lee HH, Worm M et al (2006) Development of alopecia areata universalis in a patient receiving adalimumab. Arch Dermatol 142:1654–1655
Ferran M, Calvet J, Almirall M et al (2011) Alopecia areata as another immune-mediated disease developed in patients treated with tumour necrosis factor-α blocker agents: report of five cases and review of the literature. J Eur Acad Dermatol Venereol 25:479–484
Alghamdi KM, Khurrum H, Taieb A et al (2012) Treatment of generalized vitiligo with anti-TNF-α agents. J Drugs Dermatol 11:534–539
Pelechas E, Papoudou-Bai A, Voulgari PV et al (2021) Cutaneous Autoimmune Phenomena of the Anti-TNFa Biosimilars. Casebased Review. Curr Rheumatol Rev 17:267–270
Gasparyan AY, Ayvazyan L, Blackmore H et al (2011) Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int 31:1409–1417
Strazzulla LC, Wang EHC, Avila L et al (2018) Alopecia areata: Disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol 78:1–12
Darwin E, Hirt PA, Fertig R et al (2018) Alopecia areata: review of epidemiology, clinical features, pathogenesis, and new treatment options. Int J Trichol 10:51–60
Concha JSS, Werth VP (2018) Alopecias in lupus erythematosus. Lupus Sci Med 5:e000291
Rajabi F, Drake LA, Senna MM et al (2018) Alopecia areata: a review of disease pathogenesis. Br J Dermatol 179:1033–1048
Xing L, Dai Z, Jabbari A et al (2014) Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med 20:1043–1049
Loh SH, Moon HN, Lew BL et al (2018) Role of T helper 17 cells and T regulatory cells in alopecia areata: comparison of lesion and serum cytokine between controls and patients. J Eur Acad Dermatol Venereol 32:1028–1033
Gorcey L, Gordon Spratt EA, Leger MC (2014) Alopecia universalis successfully treated with adalimumab. JAMA Dermatol 150:1341–1344
Zschoche C, Bidier M, Hadaschik E (2013) Alopecia areata during treatment with adalimumab: therapy with an alternative TNF-alpha inhibitor is possible. J Dtsch Dermatol Ges 11:450–453
Le Bidre E, Chaby G, Martin L et al (2011) Alopecia areata during anti-TNF alpha therapy: nine cases. Ann Dermatol Venereol 138:285–293
Tauber M, Buche S, Reygagne P et al (2014) Alopecia areata occurring during anti-TNF therapy: a national multicenter prospective study. J Am Acad Dermatol 70:1146–1149
Bae JM, Kim M, Lee HH et al (2018) Increased risk of vitiligo following anti-tumor necrosis factor therapy: a 10-year population-based cohort study. J Invest Dermatol 138:768–774
Palucka AK, Blanck JP, Bennett L et al (2005) Cross-regulation of TNF and IFN-alpha in autoimmune diseases. Proc Natl Acad Sci USA 102:3372–3377
Silva LC, Ortigosa LC, Benard G (2010) Anti-TNF-α agents in the treatment of immune-mediated inflammatory diseases: mechanisms of action and pitfalls. Immunotherapy 2:817–833
Acknowledgements
The authors thank Ms. Chrysa Arvaniti for her excellent secretarial assistance.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or non-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors have made a substantial contribution to the current manuscript and have approved the final version fulfilling all the four criteria requested by the ICNJE. EP: drafting, PVV revision, AAD: conception of the work, revision.
Corresponding author
Ethics declarations
Conflict of interest
All authors (E. Pelechas, PV. Voulgari & AA. Drosos) declare no conflicts of interest.
Research involving human participants and/or animals
As per our institution’s ethic’s approval, when there is no sensitive data of the patient presented (face characteristics, name/surname/date of birth on photos/x-rays/ct/mri etc.) there is no need for a specific approval.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pelechas, E., Voulgari, P.V. & Drosos, A.A. TNFα inhibitor biosimilars associated with alopecia areata. Case-based review. Rheumatol Int 42, 1113–1117 (2022). https://doi.org/10.1007/s00296-022-05129-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-022-05129-w