Abstract
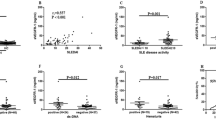
In view of the possible involvement of vascular endothelial growth factor-C (VEGF-C) in pathogenesis of adult-onset Still's disease (AOSD) based on our previous genome-wide association study (GWAS) results, the primary objective of this study, therefore, was to investigate the correlations between the content of VEGF-C in serum and clinical and biochemical markers of AOSD. Blood samples were collected from 80 patients with AOSD, 26 with rheumatoid arthritis (RA), 30 with systemic lupus erythematosus (SLE) and 31 healthy control subjects. The serum VEGF-C levels were determined using an enzyme-linked immunosorbent assay (ELISA). Statistical analysis and comparisons were conducted. A significantly higher serum VEGF-C level was observed in patients with AOSD than in HC. Serum VEGF-C levels had high AUC value of 0.8145 for distinguishing AOSD group from healthy group with sensitivity of 0.7097 and specificity of 0.8250. It also showed good diagnostic value to differentiate AOSD from other autoinflammatory diseases with sensitivity of 0.7500 and specificity of 0.5500. AOSD patients with fever, arthralgia, skin rash, sore throat, lymphadenopathy, splenomegaly hepatomegaly and pleuritis, had a higher level than those who did not have these symptoms (p = 0.0012, p = 0.0092, p = 0.0056, p = 0.0123, p = 0.0068, p = 0.0030, p = 0.0020, and p = 0.0018, respectively). The serum VEGF-C levels were also positively correlated with laboratory features and several cytokines related to AOSD disease activity. In conclusion, our study unveiled a close association between serum VEGF-C levels and AOSD including disease activity and clinical hematological manifestations, suggesting the potential utility of VEGF-C as a candidate biomarker to assess disease activity in AOSD.




Similar content being viewed by others
Abbreviations
- ANOVA:
-
One-way analysis of variance
- AOSD:
-
Adult-onset Still's disease
- AUC:
-
Area under the curve
- CRP:
-
C-reactive protein
- DMARDs:
-
Disease-modifying anti-rheumatic drugs
- ELISA:
-
Enzyme-linked immunosorbent assay
- ESR:
-
Erythrocyte sedimentation rate
- FLS:
-
Fibroblast-like synoviocytes
- GD:
-
Graves’ diseases
- GWAS:
-
Genome-wide association study
- HC:
-
Healthy control
- HLA:
-
Human leukocyte antigen
- HT:
-
Hashimoto's thyroiditis
- LDH:
-
Lactate dehydrogenase
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- ROC:
-
Receiver operating characteristic
- TNF:
-
Tumor necrosis factor
- VEGF-C:
-
Vascular endothelial growth factor-C
References
Gerfaud-Valentin M, Jamilloux Y, Iwaz J (2014) Adult-onset Still’s disease. Autoimmun Rev 13(7):708–722. https://doi.org/10.1016/j.autrev.2014.01.058
Fautrel B (2008) Adult-onset Still disease. Best Pract Res Clin Rheumatol 22(5):773–792. https://doi.org/10.1016/j.berh.2008.08.006
Feist E, Mitrovic S, Fautrel B (2018) Mechanisms, biomarkers and targets for adult-onset Still’s disease. Nat Rev Rheumatol 14(10):603–618. https://doi.org/10.1038/s41584-018-0081-x
Wouters JM, Reekers P, van de Putte LB (1986) Adult-onset Still’s disease. Disease course and HLA associations. Arthritis Rheum 29(3):415–418. https://doi.org/10.1002/art.1780290316
Miller ML, Aaron S, Jackson J, Fraser P, Cairns L, Hoch S, Borel Y, Larson M, Glass DN (1985) HLA gene frequencies in children and adults with systemic onset juvenile rheumatoid arthritis. Arthritis Rheum 28(2):146–150. https://doi.org/10.1002/art.1780280207
Asano T, Furukawa H, Sato S, Yashiro M, Kobayashi H, Watanabe H, Suzuki E, Ito T, Ubara Y, Kobayashi D, Iwanaga N, Izumi Y, Fujikawa K, Yamasaki S, Nakamura T, Koga T, Shimizu T, Umeda M, Nonaka F, Yasunami M, Ueki Y, Eguchi K, Tsuchiya N, Tohma S, Yoshiura KI, Ohira H, Kawakami A, Migita K (2017) Effects of HLA-DRB1 alleles on susceptibility and clinical manifestations in Japanese patients with adult onset Still’s disease. Arthritis Res Ther 19(1):199. https://doi.org/10.1186/s13075-017-1406-x
Fujita Y, Furukawa H, Asano T, Sato S, Yashiro Furuya M, Kobayashi H, Watanabe H, Suzuki E, Koga T, Shimizu T, Ueki Y, Eguchi K, Tsuchiya N, Kawakami A, Migita K (2019) HLA-DQB1 DPB1 alleles in Japanese patients with adult-onset Still’s disease. Mod Rheumatol 29(5):843–847. https://doi.org/10.1080/14397595.2018.1514999
Li ZQ, Liu HL, Chen JH, Zeng T, He L, Li MH, Luo CN, Liu S, Ding TT, Yimaiti K, Teng JL, Li XW, Ding YH, Cheng XB, Zhou J, Ye JN, Ji J, Su YT, Shi H, Sun Y, Gao CW, Hu QY, Chi HH, Yuan X, Zhou ZC, Wang D, Wang K, Li CG, Sun YC, Niu YJ, Chen LJ, Xu J, Wu LJ, Zhou ZW, Pan D, Niu HT, Shi YY, Yang CD (2020) Both HLA class I and II regions identified as genome-wide significant susceptibility loci for adult-onset Still’s disease in Chinese individuals. Ann Rheum Dis 79(1):161. https://doi.org/10.1136/annrheumdis-2019-215239
Secker GA, Harvey NL (2021) Regulation of VEGFR signalling in lymphatic vascular development and disease: an update. Int J Mol Sci 22(14):7760. https://doi.org/10.3390/ijms22147760
Zhan H, Li H, Liu C, Cheng L, Yan S, Li Y (2021) Association of Circulating Vascular Endothelial Growth Factor Levels with Autoimmune Diseases: a systematic review and meta-analysis. Front Immunol 12:674343. https://doi.org/10.3389/fimmu.2021.674343
Chen HK, Zhang TY, Gong BL, Cao XH (2015) Association between VEGF-634G/C polymorphism and susceptibility to autoimmune diseases: a meta-analysis. Gene 558(2):181–186. https://doi.org/10.1016/j.gene.2015.01.023
Jia W, Wu W, Yang D, Xiao C, Huang M, Long F, Su Z, Qin M, Liu X, Zhu YZ (2018) GATA4 regulates angiogenesis and persistence of inflammation in rheumatoid arthritis. Cell Death Dis 9(5):503. https://doi.org/10.1038/s41419-018-0570-5
Abdel-Maged AE, Gad AM, Wahdan SA, Azab SS (2019) Efficacy and safety of Ramucirumab and methotrexate co-therapy in rheumatoid arthritis experimental model: Involvement of angiogenic and immunomodulatory signaling. Toxicol Appl Pharmacol 380:114702. https://doi.org/10.1016/j.taap.2019.114702
Shushanov S, Bronstein M, Adelaide J, Jussila L, Tchipysheva T, Jacquemier J, Stavrovskaya A, Birnbaum D, Karamysheva A (2000) VEGFc and VEGFR3 expression in human thyroid pathologies. Int J Cancer 86(1):47–52. https://doi.org/10.1002/(sici)1097-0215(20000401)86:1%3c47::aid-ijc7%3e3.0.co;2-r
Kumpers P, David S, Haubitz M, Hellpap J, Horn R, Brocker V, Schiffer M, Haller H, Witte T (2009) The Tie2 receptor antagonist angiopoietin 2 facilitates vascular inflammation in systemic lupus erythematosus. Ann Rheum Dis 68(10):1638–1643. https://doi.org/10.1136/ard.2008.094664
Li Y, Yang B, Bai JY, Xia S, Mao M, Li X, Li N, Chen L (2019) The roles of synovial hyperplasia, angiogenesis and osteoclastogenesis in the protective effect of apigenin on collagen-induced arthritis. Int Immunopharmacol 73:362–369. https://doi.org/10.1016/j.intimp.2019.05.024
Cheng CW, Wu CZ, Tang KT, Fang WF, Lin JD (2020) Simultaneous measurement of twenty-nine circulating cytokines and growth factors in female patients with overt autoimmune thyroid diseases. Autoimmunity 53(5):261–269. https://doi.org/10.1080/08916934.2020.1755965
Yamaguchi M, Ohta A, Tsunematsu T, Kasukawa R, Mizushima Y, Kashiwagi H, Kashiwazaki S, Tanimoto K, Matsumoto Y, Ota T et al (1992) Preliminary criteria for classification of adult Still’s disease. J Rheumatol 19(3):424–430
Rau M, Schiller M, Krienke S, Heyder P, Lorenz H, Blank N (2010) Clinical manifestations but not cytokine profiles differentiate adult-onset Still’s disease and sepsis. J Rheumatol 37(11):2369–2376. https://doi.org/10.3899/jrheum.100247
Girard C, Rech J, Brown M, Allali D, Roux-Lombard P, Spertini F, Schiffrin EJ, Schett G, Manger B, Bas S, Del Val G, Gabay C (2016) Elevated serum levels of free interleukin-18 in adult-onset Still’s disease. Rheumatology 55(12):2237–2247. https://doi.org/10.1093/rheumatology/kew300
Meng J, Chi H, Wang Z, Zhang H, Sun Y, Teng J, Hu Q, Liu H, Cheng X, Ye J, Shi H, Wu X, Jia J, Wang M, Ma Y, Zhou Z, Wang F, Liu T, Wan L, Qiao X, Chen X, Yang C, Su Y (2021) Characteristics and risk factors of relapses in patients with adult-onset still’s disease: a long-term cohort study. Rheumatology. https://doi.org/10.1093/rheumatology/keab023
Grievink HW, Luisman T, Kluft C, Moerland M, Malone KE (2016) Comparison of Three Isolation Techniques for Human Peripheral Blood Mononuclear Cells: Cell Recovery and Viability, Population Composition, and Cell Functionality. Biopreserv Biobank 14(5):410–415. https://doi.org/10.1089/bio.2015.0104
Wongpiyabovorn J, Yooyongsatit S, Ruchusatsawat K, Avihingsanon Y, Hirankarn N (2008) Association of the CTG (-2578/-460/+405) haplotype within the vascular endothelial growth factor gene with early-onset psoriasis. Tissue Antigens 72(5):458–463. https://doi.org/10.1111/j.1399-0039.2008.01134.x
Sugiura T, Kawaguchi Y, Harigai M, Terajima-Ichida H, Kitamura Y, Furuya T, Ichikawa N, Kotake S, Tanaka M, Hara M, Kamatani N (2002) Association between adult-onset Still’s disease and interleukin-18 gene polymorphisms. Genes Immun 3(7):394–399. https://doi.org/10.1038/sj.gene.6363922
Kawaguchi Y, Terajima H, Harigai M, Hara M, Kamatani N (2001) Interleukin-18 as a novel diagnostic marker and indicator of disease severity in adult-onset Still’s disease. Arthritis Rheum 44(7):1716–1717. https://doi.org/10.1002/1529-0131(200107)44:7%3c1716::Aid-Art298%3e3.0.Co;2-I
Hoshino T, Ohta A, Yang D, Kawamoto M, Kikuchi M, Inoue Y, Kamizono S, Ota T, Itoh K, Oizumi K (1998) Elevated serum interleukin 6, interferon-gamma, and tumor necrosis factor-alpha levels in patients with adult Still’s disease. J Rheumatol 25(2):396–398
Chen DY, Lan JL, Lin FJ, Hsieh TY, Wen MC (2004) Predominance of Th1 cytokine in peripheral blood and pathological tissues of patients with active untreated adult onset Still’s disease. Ann Rheum Dis 63(10):1300–1306. https://doi.org/10.1136/ard.2003.013680
Koga T, Sumiyoshi R, Furukawa K, Sato S, Migita K, Shimizu T, Umeda M, Endo Y, Fukui S, Kawashiri SY, Iwamoto N, Ichinose K, Tamai M, Nakamura H, Origuchi T, Nonaka F, Yachie A, Kondo H, Maeda T, Kawakami A (2020) Interleukin-18 and fibroblast growth factor 2 in combination is a useful diagnostic biomarker to distinguish adult-onset Still’s disease from sepsis. Arthritis Res Ther 22(1):108. https://doi.org/10.1186/s13075-020-02200-4
MacDonald IJ, Liu SC, Su CM, Wang YH, Tsai CH, Tang CH (2018) Implications of angiogenesis involvement in arthritis. Int J Mol Sci 19(7):2012. https://doi.org/10.3390/ijms19072012
Osada M, Imaoka S, Funae Y (2004) Apigenin suppresses the expression of VEGF, an important factor for angiogenesis, in endothelial cells via degradation of HIF-1alpha protein. FEBS Lett 575(1–3):59–63. https://doi.org/10.1016/j.febslet.2004.08.036
Wauke K, Nagashima M, Ishiwata T, Asano G, Yoshino S (2002) Expression and localization of vascular endothelial growth factor-C in rheumatoid arthritis synovial tissue. J Rheumatol 29(1):34–38
Paavonen K, Mandelin J, Partanen T, Jussila L, Li TF, Ristimaki A, Alitalo K, Konttinen YT (2002) Vascular endothelial growth factors C and D and their VEGFR-2 and 3 receptors in blood and lymphatic vessels in healthy and arthritic synovium. J Rheumatol 29(1):39–45
Cha HS, Bae EK, Koh JH, Chai JY, Jeon CH, Ahn KS, Kim J, Koh EM (2007) Tumor necrosis factor-alpha induces vascular endothelial growth factor-C expression in rheumatoid synoviocytes. J Rheumatol 34(1):16–19
Bouta EM, Bell RD, Rahimi H, Xing LP, Wood RW, Bingham CO, Ritchlin CT, Schwarz EM (2018) Targeting lymphatic function as a novel therapeutic intervention for rheumatoid arthritis. Nat Rev Rheumatol 14(2):94–106. https://doi.org/10.1038/nrrheum.2017.205
Palazon A, Tyrakis PA, Macias D, Velica P, Rundqvist H, Fitzpatrick S, Vojnovic N, Phan AT, Loman N, Hedenfalk I, Hatschek T, Lovrot J, Foukakis T, Goldrath AW, Bergh J, Johnson RS (2017) An HIF-1alpha/VEGF-A Axis in Cytotoxic T Cells Regulates Tumor Progression. Cancer Cell 32(5):669-683.e665. https://doi.org/10.1016/j.ccell.2017.10.003
Clauss M, Gerlach M, Gerlach H, Brett J, Wang F, Familletti PC, Pan YC, Olander JV, Connolly DT, Stern D (1990) Vascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J Exp Med 172(6):1535–1545. https://doi.org/10.1084/jem.172.6.1535
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9(6):669–676. https://doi.org/10.1038/nm0603-669
Ferrara N, Davis-Smyth T (1997) The biology of vascular endothelial growth factor. Endocr Rev 18(1):4–25. https://doi.org/10.1210/edrv.18.1.0287
Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ (1996) Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem 271(2):736–741. https://doi.org/10.1074/jbc.271.2.736
Kwon YW, Kwon KS, Moon HE, Park JA, Choi KS, Kim YS, Jang HS, Oh CK, Lee YM, Kwon YG, Lee YS, Kim KW (2004) Insulin-like growth factor-II regulates the expression of vascular endothelial growth factor by the human keratinocyte cell line HaCaT. J Invest Dermatol 123(1):152–158. https://doi.org/10.1111/j.0022-202X.2004.22735.x
Malaguarnera L, Imbesi R, Di Rosa M, Scuto A, Castrogiovanni P, Messina A, Sanfilippo S (2005) Action of prolactin, IFN-gamma, TNF-alpha and LPS on heme oxygenase-1 expression and VEGF release in human monocytes/macrophages. Int Immunopharmacol 5(9):1458–1469. https://doi.org/10.1016/j.intimp.2005.04.002
Valter MM, Wiestler OD, Pietsche T (1999) Differential control of VEGF synthesis and secretion in human glioma cells by IL-1 and EGF. Int J Dev Neurosci 17(5–6):565–577. https://doi.org/10.1016/s0736-5748(99)00048-9
McNab F, Mayer-Barber K, Sher A, Wack A, O’Garra A (2015) Type I interferons in infectious disease. Nat Rev Immunol 15(2):87–103. https://doi.org/10.1038/nri3787
Boxx GM, Cheng G (2016) The Roles of Type I Interferon in Bacterial Infection. Cell Host Microbe 19(6):760–769. https://doi.org/10.1016/j.chom.2016.05.016
Hall JC, Rosen A (2010) Type I interferons: crucial participants in disease amplification in autoimmunity. Nat Rev Rheumatol 6(1):40–49. https://doi.org/10.1038/nrrheum.2009.237
Postal M, Vivaldo JF, Fernandez-Ruiz R, Paredes JL, Appenzeller S, Niewold TB (2020) Type I interferon in the pathogenesis of systemic lupus erythematosus. Curr Opin Immunol 67:87–94. https://doi.org/10.1016/j.coi.2020.10.014
Ning F, Li X, Yu L, Zhang B, Zhao Y, Liu Y, Zhao B, Shang Y, Hu X (2019) Hes1 attenuates type I IFN responses via VEGF-C and WDFY1. J Exp Med 216(6):1396–1410. https://doi.org/10.1084/jem.20180861
Acknowledgements
We thank all the patients who were involved in the study.
Funding
This research was funded by National Natural Science Foundation of China (81502016).
Author information
Authors and Affiliations
Contributions
Conceptualization, CY and HLL; data curation, XC and QH; formal analysis, HLL; methodology, QH; project administration, HLL; resources, MW, JJ, JT, YS, XC, JY, YS, HS, HC, ZZ, TL, ZW, LW, XQ, FW and XW; supervision, CY and HLL; writing original draft, XC and QH.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
The studies involving human participants were reviewed and approved by the Institutional Research Ethics Committee of Ruijin Hospital (identifier 2016–62), Shanghai, China. The patients/participants provided their written informed consent to participate in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, X., Hu, Qy., Wang, M. et al. Serum VEGF-C as an evaluation marker of disease activity in adult-onset Still's disease. Rheumatol Int 42, 149–157 (2022). https://doi.org/10.1007/s00296-021-04978-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-021-04978-1