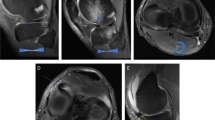
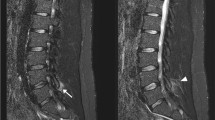
Abstract
Peripheral neuropathy in patients with rheumatoid arthritis is associated with a maladaptive autoimmune response that may cause chronic pain and disability. Nerve conduction studies are the routine method performed when rheumatologists presume its presence. However, this approach is invasive, may not reveal subtle malfunctions in the early stages of the disease, and does not expose abnormalities in structures surrounding the nerves and muscles, limiting the possibility of a timely diagnosis. This work aims to present a narrative review of new technologies for the clinical assessment of peripheral neuropathy in Rheumatoid Arthritis. Through a bibliographic search carried out in five repositories, from 1990 to 2020, we identified three technologies that could detect peripheral nerve lesions and perform quantitative evaluations: (1) magnetic resonance neurography, (2) functional magnetic resonance imaging, and (3) high-resolution ultrasonography of peripheral nerves. We found these tools can overcome the main constraints imposed by the previous electrophysiologic methods, enabling early diagnosis.
Similar content being viewed by others
Abbreviations
- PN:
-
Peripheral neuropathy
- RA:
-
Rheumatoid arthritis
- NCS:
-
Nerve conduction studies
- MRI:
-
Magnetic resonance imaging
- MR:
-
Magnetic resonance
- MRN:
-
Magnetic resonance neurography
- SNR:
-
Signal-to-noise ratio
- DWI:
-
Diffusion-weighted imaging
- DTI:
-
Diffusion tensor imaging
- FA:
-
Fractional anisotropy
- fMRI:
-
Functional magnetic resonance imaging
- BOLD:
-
Blood oxygenation-level dependent
- CIDP:
-
Chronic inflammatory demyelinating polyneuropathy
- TNS:
-
Total neuropathy score
- US:
-
Ultrasound
- HRUS:
-
High-resolution ultrasonography
- GSUS:
-
Greyscale US
- DPUS:
-
Power Doppler US
- TNF:
-
Tumour necrosis factor
References
Reda H, Chin RL (2014) Peripheral neuropathies of rheumatologic disease and gluten-related disorders. Semin Neurol 34(4):413–424. https://doi.org/10.1055/s-0034-1390390
Biswas M, Chatterjee A, Ghosh SK, Dasgupta S, Ghosh K, Ganguly PK (2011) Prevalence, types, clinical associations, and determinants of peripheral neuropathy in rheumatoid patients. Ann Indian Acad Neurol 14(3):194–197. https://doi.org/10.4103/0972-2327.85893
Sivri A, Güler-Uysal F (1999) The electroneurophysiological findings in rheumatoid arthritis patients. Electromyogr Clin Neurophysiol 39(7):387–391
Kaeley N, Ahmad S, Pathania M, Kakkar R (2019) Prevalence and patterns of peripheral neuropathy in patients of rheumatoid arthritis. J Family Med Prim Care 8(1):22–26. https://doi.org/10.4103/jfmpc.jfmpc_260_18
Cojocaru M, Cojocaru IM, Silosi I, Vrabie CD, Tanasescu R (2010) Extra-articular Manifestations in Rheumatoid Arthritis. Maedica 5(4):286–291
Bayrak AO, Durmus D, Durmaz Y, Demir I, Canturk F, Onar MK (2010) Electrophysiological assessment of polyneuropathic involvement in rheumatoid arthritis: relationships among demographic, clinical and laboratory findings. Neurol Res 32(7):711–714. https://doi.org/10.1179/016164109x12581096870195
Vallat JM, Rabin M, Magy L (2012) Peripheral neuropathies in rheumatic disease—a guide to diagnosis. Nat Rev Rheumatol 8(10):599–609. https://doi.org/10.1038/nrrheum.2012.138
Salaffi F, Giacobazzi G, Di Carlo M (2018) Chronic pain in inflammatory arthritis: mechanisms, metrology, and emerging targets—a focus on the JAK-STAT pathway. Pain Res Manag 2018:8564215. https://doi.org/10.1155/2018/8564215
Courvoisier N, Dougados M, Cantagrel A, Goupille P, Meyer O, Sibilia J, Daures JP, Combe B (2008) Prognostic factors of 10-year radiographic outcome in early rheumatoid arthritis: a prospective study. Arthritis Res Ther 10(5):R106. https://doi.org/10.1186/ar2498
Atzeni F, Cazzola M, Benucci M, Di Franco M, Salaffi F, Sarzi-Puttini P (2011) Chronic widespread pain in the spectrum of rheumatological diseases. Best Pract Res Clin Rheumatol 25(2):165–171. https://doi.org/10.1016/j.berh.2010.01.011
Mallik A, Weir AI (2005) Nerve conduction studies: essentials and pitfalls in practice. J Neurol Neurosurg Psychiatry 76(Suppl 2):ii23-31. https://doi.org/10.1136/jnnp.2005.069138
Bromberg MB (2013) An electrodiagnostic approach to the evaluation of peripheral neuropathies. Phys Med Rehabil Clin N Am 24(1):153–168. https://doi.org/10.1016/j.pmr.2012.08.020
Chhabra A, Andreisek G, Soldatos T, Wang KC, Flammang AJ, Belzberg AJ, Carrino JA (2011) MR neurography: past, present, and future. AJR Am J Roentgenol 197(3):583–591. https://doi.org/10.2214/ajr.10.6012
Magda P, Latov N, Renard MV, Sander HW (2002) Quantitative sensory testing: high sensitivity in small fiber neuropathy with normal NCS/EMG. J Peripher Nerv Syst 7(4):225–228. https://doi.org/10.1046/j.1529-8027.2002.02029.x
Casteleyn V, Hahn K, Stenzel W, Siegert E (2019) Peripheral nerve involvement in rheumatic diseases. Z Rheumatol 78(4):339–351. https://doi.org/10.1007/s00393-019-0621-z
Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD (2011) Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int 31(11):1409–1417. https://doi.org/10.1007/s00296-011-1999-3
Schardt C, Adams MB, Owens T, Keitz S, Fontelo P (2007) Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak 7:16. https://doi.org/10.1186/1472-6947-7-16
Chapter 6—Magnetic resonance imaging (2011). In: Waldman SD, Campbell RSD (eds) Imaging of Pain. W.B. Saunders Philadelphia, pp. 19–21. https://doi.org/10.1016/B978-1-4377-0906-3.00006-7
Chou ET, Carrino JA (2007) Magnetic resonance imaging. In: Waldman SD (ed) Pain management. W.B. Saunders, Philadelphia, pp. 106–117. https://doi.org/10.1016/B978-0-7216-0334-6.50014-5
Chen Y, Haacke EM, Li J (2019) Peripheral nerve magnetic resonance imaging. F1000Research 8:1803. https://doi.org/10.12688/f1000research.19695.1
Martinez AR, Nunes MB, Nucci A, França MC Jr (2012) Sensory neuronopathy and autoimmune diseases. Autoimmune Dis 2012:873587. https://doi.org/10.1155/2012/873587
Kronlage M, Schwehr V, Schwarz D, Godel T, Heiland S, Bendszus M, Bäumer P (2019) Magnetic resonance neurography : normal values and demographic determinants of nerve caliber and T2 relaxometry in 60 healthy individuals. Clin Neuroradiol 29(1):19–26. https://doi.org/10.1007/s00062-017-0633-5
Hargreaves BA, Worters PW, Pauly KB, Pauly JM, Koch KM, Gold GE (2011) Metal-induced artifacts in MRI. AJR Am J Roentgenol 197(3):547–555. https://doi.org/10.2214/ajr.11.7364
Chhabra A, Madhuranthakam AJ, Andreisek G (2018) Magnetic resonance neurography: current perspectives and literature review. Eur Radiol 28(2):698–707. https://doi.org/10.1007/s00330-017-4976-8
Bendszus M, Wessig C, Solymosi L, Reiners K, Koltzenburg M (2004) MRI of peripheral nerve degeneration and regeneration: correlation with electrophysiology and histology. Exp Neurol 188(1):171–177. https://doi.org/10.1016/j.expneurol.2004.03.025
Filler AG, Howe FA, Hayes CE, Kliot M, Winn HR, Bell BA, Griffiths JR, Tsuruda JS (1993) Magnetic resonance neurography. Lancet 341(8846):659–661. https://doi.org/10.1016/0140-6736(93)90422-d
Dailey AT, Tsuruda JS, Filler AG, Maravilla KR, Goodkin R, Kliot M (1997) Magnetic resonance neurography of peripheral nerve degeneration and regeneration. Lancet 350(9086):1221–1222. https://doi.org/10.1016/s0140-6736(97)24043-2
Chhabra A, Lee PP, Bizzell C, Soldatos T (2011) 3 Tesla MR neurography—technique, interpretation, and pitfalls. Skeletal Radiol 40(10):1249–1260. https://doi.org/10.1007/s00256-011-1183-6
Stoll G, Wilder-Smith E, Bendszus M (2013) Imaging of the peripheral nervous system. Handb Clin Neurol 115:137–153. https://doi.org/10.1016/b978-0-444-52902-2.00008-4
Husarik DB, Saupe N, Pfirrmann CW, Jost B, Hodler J, Zanetti M (2009) Elbow nerves: MR findings in 60 asymptomatic subjects—normal anatomy, variants, and pitfalls. Radiology 252(1):148–156. https://doi.org/10.1148/radiol.2521081614
Deshmane A, Gulani V, Griswold MA, Seiberlich N (2012) Parallel MR imaging. J Magn Reson Imaging 36(1):55–72. https://doi.org/10.1002/jmri.23639
Chhabra A, Zhao L, Carrino JA, Trueblood E, Koceski S, Shteriev F, Lenkinski L, Sinclair CD, Andreisek G (2013) MR neurography: advances. Radiol Res Pract 2013:809568. https://doi.org/10.1155/2013/809568
Andersson G, Orädd G, Sultan F, Novikov LN (2018) In vivo diffusion tensor imaging, diffusion kurtosis imaging, and tractography of a sciatic nerve injury model in rat at 9.4 T. Sci Rep 8(1):12911. https://doi.org/10.1038/s41598-018-30961-1
Zhu LH, Zhang ZP, Wang FN, Cheng QH, Guo G (2019) Diffusion kurtosis imaging of microstructural changes in brain tissue affected by acute ischemic stroke in different locations. Neural Regen Res 14(2):272–279. https://doi.org/10.4103/1673-5374.244791
Ohana M, Moser T, Moussaouï A, Kremer S, Carlier RY, Liverneaux P, Dietemann JL (2014) Current and future imaging of the peripheral nervous system. Diagn Interv Imaging 95(1):17–26. https://doi.org/10.1016/j.diii.2013.05.008
Martín Noguerol T, Barousse R, Gómez Cabrera M, Socolovsky M, Bencardino JT, Luna A (2019) Functional MR neurography in evaluation of peripheral nerve trauma and postsurgical assessment. Radiographics 39(2):427–446. https://doi.org/10.1148/rg.2019180112
Heckel A, Weiler M, Xia A, Ruetters M, Pham M, Bendszus M, Heiland S, Baeumer P (2015) Peripheral nerve diffusion tensor imaging: assessment of axon and myelin sheath integrity. PLoS ONE 10(6):e0130833. https://doi.org/10.1371/journal.pone.0130833
Gersing AS, Cervantes B, Knebel C, Schwaiger BJ, Kirschke JS, Weidlich D, Claudi C, Peeters JM, Pfeiffer D, Rummeny EJ, Karampinos DC, Woertler K (2020) Diffusion tensor imaging and tractography for preoperative assessment of benign peripheral nerve sheath tumors. Eur J Radiol 129:109110. https://doi.org/10.1016/j.ejrad.2020.109110
Simon NG, Kliot M (2014) Diffusion weighted MRI and tractography for evaluating peripheral nerve degeneration and regeneration. Neural Regen Res 9(24):2122–2124. https://doi.org/10.4103/1673-5374.147941
Jambawalikar S, Baum J, Button T, Li H, Geronimo V, Gould ES (2010) Diffusion tensor imaging of peripheral nerves. Skeletal Radiol 39(11):1073–1079. https://doi.org/10.1007/s00256-010-0974-5
Bäumer P, Pham M, Ruetters M, Heiland S, Heckel A, Radbruch A, Bendszus M, Weiler M (2014) Peripheral neuropathy: detection with diffusion-tensor imaging. Radiology 273(1):185–193. https://doi.org/10.1148/radiol.14132837
Khalil C, Budzik JF, Kermarrec E, Balbi V, Le Thuc V, Cotten A (2010) Tractography of peripheral nerves and skeletal muscles. Eur J Radiol 76(3):391–397. https://doi.org/10.1016/j.ejrad.2010.03.012
Sánchez-González J, Luna A (2012) DWI at 3 T: advantages, disadvantages, pitfalls, and advanced clinical applications. Diffusion MRI outside the brain. Springer, Berlin, pp 51–73
Agarwal A, Chandra A, Jaipal U, Bagarhatta M, Mendiratta K, Goyal A, Kumar R, Mangalhara N (2019) Can imaging be the new yardstick for diagnosing peripheral neuropathy?—a comparison between high resolution ultrasound and MR neurography with an approach to diagnosis. Insights Imaging 10(1):104. https://doi.org/10.1186/s13244-019-0787-6
Pérez-Neri I, González-Aguilar A, Sandoval H, Pineda C, Ríos C (2020) Therapeutic potential of ultrasound neuromodulation in decreasing neuropathic pain: clinical and experimental evidence. Curr Neuropharmacol. https://doi.org/10.2174/1570159x18666200720175253
Dou Z, Yang L (2019) The application of functional magnetic resonance imaging in neuropathic pain. In: Medical imaging-principles and applications. IntechOpen, UK
Matthews PM, Jezzard P (2004) Functional magnetic resonance imaging. J Neurol Neurosurg Psychiatry 75(1):6–12
Brown GG, Perthen JE, Liu TT, Buxton RB (2007) A primer on functional magnetic resonance imaging. Neuropsychol Rev 17(2):107–125. https://doi.org/10.1007/s11065-007-9028-8
Boland EG, Selvarajah D, Hunter M, Ezaydi Y, Tesfaye S, Ahmedzai SH, Snowden JA, Wilkinson ID (2014) Central pain processing in chronic chemotherapy-induced peripheral neuropathy: a functional magnetic resonance imaging study. PLoS ONE 9(5):e96474. https://doi.org/10.1371/journal.pone.0096474
Moisset X, Bouhassira D (2007) Brain imaging of neuropathic pain. Neuroimage 37(Suppl 1):S80-88. https://doi.org/10.1016/j.neuroimage.2007.03.054
Endo T, Spenger C, Hao J, Tominaga T, Wiesenfeld-Hallin Z, Olson L, Xu XJ (2008) Functional MRI of the brain detects neuropathic pain in experimental spinal cord injury. Pain 138(2):292–300. https://doi.org/10.1016/j.pain.2007.12.017
Li J, Zhang W, Wang X, Yuan T, Liu P, Wang T, Shen L, Huang Y, Li N, You H, Xiao T, Feng F, Ma C (2018) Functional magnetic resonance imaging reveals differences in brain activation in response to thermal stimuli in diabetic patients with and without diabetic peripheral neuropathy. PLoS ONE 13(1):e0190699. https://doi.org/10.1371/journal.pone.0190699
Yeh CH, Caswell K, Pandiri S, Sair H, Lukkahatai N, Campbell CM, Stearns V, Van de Castle B, Perrin N, Smith TJ, Saligan LN (2020) Dynamic brain activity following auricular point acupressure in chemotherapy-induced neuropathy: a pilot longitudinal functional magnetic resonance imaging study. Glob Adv Health Med 9:2164956120906092. https://doi.org/10.1177/2164956120906092
Baumgärtner U, Iannetti GD, Zambreanu L, Stoeter P, Treede RD, Tracey I (2010) Multiple somatotopic representations of heat and mechanical pain in the operculo-insular cortex: a high-resolution fMRI study. J Neurophysiol 104(5):2863–2872. https://doi.org/10.1152/jn.00253.2010
Hsieh ST (2010) Pathology and functional diagnosis of small-fiber painful neuropathy. Acta Neurol Taiwan 19(2):82–89
Borsook D, Becerra L (2007) Phenotyping central nervous system circuitry in chronic pain using functional MRI: considerations and potential implications in the clinic. Curr Pain Headache Rep 11(3):201–207. https://doi.org/10.1007/s11916-007-0191-7
Fomberstein K, Qadri S, Ramani R (2013) Functional MRI and pain. Curr Opin Anesthesiol 26(5):588–593. https://doi.org/10.1097/01.aco.0000433060.59939.fe
Hofbauer RK, Olausson HW, Bushnell MC (2006) Thermal and tactile sensory deficits and allodynia in a nerve-injured patient: a multimodal psychophysical and functional magnetic resonance imaging study. Clin J Pain 22(1):104–108. https://doi.org/10.1097/01.ajp.0000149798.93498.7c
Padua L, Liotta G, Di Pasquale A, Granata G, Pazzaglia C, Caliandro P, Martinoli C (2012) Contribution of ultrasound in the assessment of nerve diseases. Eur J Neurol 19(1):47–54. https://doi.org/10.1111/j.1468-1331.2011.03421.x
Di Pasquale A, Morino S, Loreti S, Bucci E, Vanacore N, Antonini G (2015) Peripheral nerve ultrasound changes in CIDP and correlations with nerve conduction velocity. Neurology 84(8):803–809. https://doi.org/10.1212/wnl.0000000000001291
Wilder-Smith EP, Rajendran K, Therimadasamy AK (2009) High-resolution ultrasonography for peripheral nerve diagnostics: a guide for clinicians involved in diagnosis and management of peripheral nerve disorders. World Scientific, Singapore
Moritz T, Prosch H, Pivec CH, Sachs A, Pretterklieber ML, Kriechbaumer L, Happak W, Bodner G (2014) High-resolution ultrasound visualization of the subcutaneous nerves of the forearm: a feasibility study in anatomic specimens. Muscle Nerve 49(5):676–679. https://doi.org/10.1002/mus.24064
Chavez-Lopez MA, Hernandez-Diaz C, Moya C, Pineda C, Ventura-Rios L, Moller I, Naredo E, Espinosa R, Pena A, Rosas-Cabral A, Filippucci E (2013) Inter- and intra-observer agreement of high-resolution ultrasonography and power Doppler in assessment of joint inflammation and bone erosions in patients with rheumatoid arthritis. Rheumatol Int 33(1):173–177. https://doi.org/10.1007/s00296-011-2297-9
Gallardo E, Noto Y, Simon NG (2015) Ultrasound in the diagnosis of peripheral neuropathy: structure meets function in the neuromuscular clinic. J Neurol Neurosurg Psychiatry 86(10):1066–1074. https://doi.org/10.1136/jnnp-2014-309599
Blanch S, Demondion X, Bard H, Montet X, Martinoli C (2007) Échographie du nerf médian. Rev rhum (Ed française) 74(4):376–383
Üçeyler N, Schäfer KA, Mackenrodt D, Sommer C, Müllges W (2016) High-resolution ultrasonography of the superficial peroneal motor and sural sensory nerves may be a non-invasive approach to the diagnosis of vasculitic neuropathy. Front Neurol 7:48. https://doi.org/10.3389/fneur.2016.00048
El-Karabaty H, Hetzel A, Galla TJ, Horch RE, Lücking CH, Glocker FX (2005) The effect of carpal tunnel release on median nerve flattening and nerve conduction. Electromyogr Clin Neurophysiol 45(4):223–227
Ginn SD, Cartwright MS, Chloros GD, Walker FO, Yoon JS, Brown ME, Wiesler ER (2007) Ultrasound in the diagnosis of a median neuropathy in the forearm: case report. J Brachial Plex Peripher Nerve Inj 2:23. https://doi.org/10.1186/1749-7221-2-23
Cartwright MS, Chloros GD, Walker FO, Wiesler ER, Campbell WW (2007) Diagnostic ultrasound for nerve transection. Muscle Nerve 35(6):796–799. https://doi.org/10.1002/mus.20761
Peer S, Harpf C, Willeit J, Piza-Katzer H, Bodner G (2003) Sonographic evaluation of primary peripheral nerve repair. J Ultrasound Med 22(12):1317–1322. https://doi.org/10.7863/jum.2003.22.12.1317
Chhabra A, Subhawong TK, Andreisek G (2012) Magnetic resonance neurography of tunnels—part I: upper extremity nerves. In: Magnetic resonance neurography. pp. 37
Beekman R, Van Der Plas JP, Uitdehaag BM, Schellens RL, Visser LH (2004) Clinical, electrodiagnostic, and sonographic studies in ulnar neuropathy at the elbow. Muscle Nerve 30(2):202–208. https://doi.org/10.1002/mus.20093
Pál E, Fülöp K, Tóth P, Deli G, Pfund Z, Janszky J, Komoly S (2020) Small fiber neuropathy: clinicopathological correlations. Behav Neurol 2020:8796519. https://doi.org/10.1155/2020/8796519
Lauria G, Merkies IS, Faber CG (2012) Small fibre neuropathy. Curr Opin Neurol 25(5):542–549. https://doi.org/10.1097/WCO.0b013e32835804c5
Hoeijmakers JG, Faber CG, Lauria G, Merkies IS, Waxman SG (2012) Small-fibre neuropathies—advances in diagnosis, pathophysiology and management. Nat Rev Neurol 8(7):369–379. https://doi.org/10.1038/nrneurol.2012.97
Camdessanché JP, Jousserand G, Ferraud K, Vial C, Petiot P, Honnorat J, Antoine JC (2009) The pattern and diagnostic criteria of sensory neuronopathy: a case–control study. Brain 132(Pt 7):1723–1733. https://doi.org/10.1093/brain/awp136
Lauria G, Sghirlanzoni A, Lombardi R, Pareyson D (2001) Epidermal nerve fiber density in sensory ganglionopathies: clinical and neurophysiologic correlations. Muscle Nerve 24(8):1034–1039. https://doi.org/10.1002/mus.1107
Jusufović E, Sinanović O, Zukić S, Burina A, Džinić Jusufović Z, Šakić A (2018) Multifocal motor neuropathy: case reports. Acta Clin Croat 57(3):581–587. https://doi.org/10.20471/acc.2018.57.03.23
Nobile-Orazio E (2001) Multifocal motor neuropathy. J Neuroimmunol 115(1–2):4–18. https://doi.org/10.1016/s0165-5728(01)00266-1
Van Asseldonk JT, Franssen H, Van den Berg-Vos RM, Wokke JH, Van den Berg LH (2005) Multifocal motor neuropathy. Lancet Neurol 4(5):309–319. https://doi.org/10.1016/s1474-4422(05)70074-0
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
CP, HS, IPN AA, JCLH and JG has been involved in writing, compiling and revising the manuscript critically to suit publication standards. All authors read and approved the final version of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest/competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable (the present paper is a review article; it describes published data).
Code availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gutiérrez, J., Sandoval, H., Pérez-Neri, I. et al. Advances in imaging technologies for the assessment of peripheral neuropathies in rheumatoid arthritis. Rheumatol Int 41, 519–528 (2021). https://doi.org/10.1007/s00296-020-04780-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-020-04780-5