Abstract
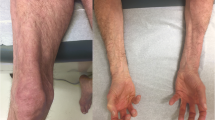
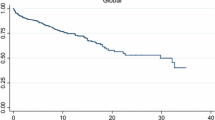
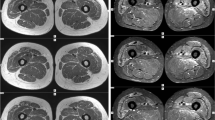
Overlap myositis (OM), an important subset of idiopathic inflammatory myopathies (IIM), is being increasingly recognized with wider myositis-specific autoantibody (MSA) testing. We studied the differences in clinical characteristics and long-term outcomes of OM with Dermatomyositis (DM), Polymyositis (PM), anti-synthetase syndrome (ASSD), and Cancer-associated IIM (CAM). Data from the MyoCite registry (Dec2017–May2020), a prospective dataset of IIM was extracted for the clinical profile, and MSAs, immunosuppressants received, disease activity (relapses and incomplete response), and treatment-related (drugs ADRs and infections) adverse events (DRAE and TRAE) were collected and analyzed between groups. Of 214 adults (58-OM,89-DM,27-ASSD,33-PM,7-CAM), OM had a greater female preponderance (13.5:1). Raynaud’s and sclerodactyly were the prime distinguishing features of OM. OM could be distinguished from PM by frequent arthritis (OR-3.2) and infrequent dysphagia (OR-0.17); DM with greater nephritis (OR-20), infrequent dysphagia (OR-0.24) and rashes (OR-0.02); and ASSD by infrequent ILD (OR-0.07), and mechanic’s hand (OR-0.05). 50% fulfilled the classification criteria for ASSD in the absence of MSA testing. ANA was positive more often (PM/DM: OR-6.7) and anti-Ro52 (OR-4.5) frequent in OM. Baseline serum creatinine and acute phase reactants were higher. OM received lower glucocorticoids (0 mg/kg, p < 0.001). Overall, 90% and 84% of OM at 12 and 24 months, respectively, achieved remission, with similar DRAE and TRAE as other IIM subsets. OM can be misdiagnosed as ASSD in the absence of MSA testing. Raynaud’s, sclerodactyly, and a positive ANA may identify OM and prevent overtreatment.

Similar content being viewed by others
Data availability statement
The data underlying this article will be shared on a reasonable request to the corresponding author.
References
Betteridge Z, Tansley S, Shaddick G et al (2019) Frequency, mutual exclusivity and clinical associations of myositis autoantibodies in a combined European cohort of idiopathic inflammatory myopathy patients. J Autoimmun 101:48–55. https://doi.org/10.1016/j.jaut.2019.04.001
McHugh NJ, Tansley SL (2018) Autoantibodies in myositis. Nat Rev Rheumatol 14:290–302. https://doi.org/10.1038/nrrheum.2018.56
Witt LJ, Curran JJ, Strek ME (2016) The diagnosis and treatment of antisynthetase syndrome. Clin Pulm Med 23:218–226. https://doi.org/10.1097/CPM.0000000000000171
Colafrancesco S, Priori R, Valesini G (2015) Inflammatory myopathies and overlap syndromes: Update on histological and serological profile. Best Pract Res Clin Rheumatol 29:810–825. https://doi.org/10.1016/j.berh.2016.02.005
Fredi M, Cavazzana I, Franceschini F (2018) The clinico-serological spectrum of overlap myositis. Curr Opin Rheumatol 30:637–643. https://doi.org/10.1097/BOR.0000000000000536
Aguila LA, Lopes MRU, Pretti FZ et al (2014) Clinical and laboratory features of overlap syndromes of idiopathic inflammatory myopathies associated with systemic lupus erythematosus, systemic sclerosis, or rheumatoid arthritis. Clin Rheumatol 33:1093–1098. https://doi.org/10.1007/s10067-014-2730-z
Maundrell A, Proudman S, Limaye V (2019) Prevalence of other connective tissue diseases in idiopathic inflammatory myopathies. Rheumatol Int 39:1777–1781. https://doi.org/10.1007/s00296-019-04411-8
Jury EC, D’Cruz D, Morrow WJW (2001) Autoantibodies and overlap syndromes in autoimmune rheumatic disease. J Clin Pathol 54:340–347. https://doi.org/10.1136/jcp.54.5.340
Lepreux S, Hainfellner JA, Vital A (2018) Idiopathic inflammatory myopathies overlapping with systemic diseases. Clin Neuropathol 37:6–15. https://doi.org/10.5414/NP301077
Rothwell S, Chinoy H, Lamb JA et al (2019) Focused HLA analysis in Caucasians with myositis identifies significant associations with autoantibody subgroups. Ann Rheum Dis 78:996–1002. https://doi.org/10.1136/annrheumdis-2019-215046
Chinoy H, Payne D, Poulton KV et al (2009) HLA–DPB1 associations differ between DRB1*03 positive anti-Jo-1 and anti-PM-Scl antibody positive idiopathic inflammatory myopathy. Rheumatology 48:1213–1217. https://doi.org/10.1093/rheumatology/kep248
Lega J-C, Cottin V, Fabien N et al (2010) Interstitial lung disease associated with Anti-PM/Scl or Anti-Aminoacyl-tRNA synthetase autoantibodies: a similar condition? J Rheumatol 37:1000–1009. https://doi.org/10.3899/jrheum.090652
Nuño-Nuño L, Joven BE, Carreira PE et al (2019) Overlap myositis, a distinct entity beyond primary inflammatory myositis: A retrospective analysis of a large cohort from the REMICAM registry. Int J Rheum Dis 22:1393–1401. https://doi.org/10.1111/1756-185X.13559
Gupta L, Appani S, Janardana R et al (2019) Meeting report: MyoIN–Pan-India collaborative network for myositis research. Indian J Rheumatol 14:136. https://doi.org/10.4103/injr.injr_40_19
Naveen R, Anuja A, Rai M et al (2020) Development of the myocite biobank: Cost-efficient model of public sector investigator-driven biobank for idiopathic inflammatory myositis. Indian J Rheumatol. https://doi.org/10.4103/injr.injr_95_20
Gupta L, Majumder S, Aggarwal A, et al. (Ahead of Print) Serum Fatty Acid‑Binding Protein 3 Levels Differentiate Active from. Indian J Rheumatol. Doi: https://doi.org/10.4103/injr.injr_57_20
Lundberg IE, Tjärnlund A, Bottai M et al (2017) EULAR/ACR classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis 76:1955–1964. https://doi.org/10.1136/annrheumdis-2017-211468
STROBE Statement: https://www.strobe-statement.org/index.php?id=available-checklists. Accessed 13 Oct 2020
Petri M, Orbai A-M, Alarcón GS et al (2012) Derivation and validation of systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 64:2677–2686. https://doi.org/10.1002/art.34473
John KJ, Sadiq M, George T et al (2020) Clinical and immunological profile of mixed connective tissue disease and a comparison of four diagnostic criteria. Int J Rheumatol 2020:e9692030. https://doi.org/10.1155/2020/9692030
KAHN M-F, (1990) Syndrome de sharp. Syndr Sharp 40:1944–1945
van den Hoogen F, Khanna D, Fransen J et al (2013) 2013 Classification criteria for systemic sclerosis: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative: ACR/EULAR Classification Criteria for SSc. Arthritis Rheum 65:2737–2747. https://doi.org/10.1002/art.38098
Le Goff M, Cornec D, Jousse-Joulin S et al (2017) Comparison of 2002 AECG and 2016 ACR/EULAR classification criteria and added value of salivary gland ultrasonography in a patient cohort with suspected primary Sjögren’s syndrome. Arthritis Res Ther. https://doi.org/10.1186/s13075-017-1475-x
Connors GR, Christopher-Stine L, Oddis CV, Danoff SK (2010) Interstitial lung disease associated with the idiopathic inflammatory myopathies: what progress has been made in the past 35 years? Chest 138:1464–1474. https://doi.org/10.1378/chest.10-0180
Kaneko Y, Nunokawa T, Taniguchi Y et al (2020) Clinical characteristics of cancer-associated myositis complicated by interstitial lung disease: a large-scale multicentre cohort study. Rheumatology 59:112–119. https://doi.org/10.1093/rheumatology/kez238
Mehta P, Gupta L (2020) Combined case record forms for collaborative datasets of patients and controls of idiopathic inflammatory myopathies. Indian J Rheumatol Ahead of Print. Doi: https://doi.org/10.4103/injr.injr_56_20
Platteel ACM, Wevers BA, Lim J et al (2019) Frequencies and clinical associations of myositis-related antibodies in The Netherlands: a one-year survey of all Dutch patients. J Transl Autoimmun 2:100013. https://doi.org/10.1016/j.jtauto.2019.100013
Isenberg DA, Allen E, Farewell V et al (2004) International consensus outcome measures for patients with idiopathic inflammatory myopathies. Development and initial validation of myositis activity and damage indices in patients with adult onset disease. Rheumatol Oxf 43:49–54. https://doi.org/10.1093/rheumatology/keg427
Lilleker JB, Vencovsky J, Wang G et al (2018) The EuroMyositis registry: an international collaborative tool to facilitate myositis research. Ann Rheum Dis 77:30–39. https://doi.org/10.1136/annrheumdis-2017-211868
Yang W, Wang X, Zhang W et al (2017) Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio are 2 new inflammatory markers associated with pulmonary involvement and disease activity in patients with dermatomyositis. Clin Chim Acta Int J Clin Chem 465:11–16. https://doi.org/10.1016/j.cca.2016.12.007
Ha Y-J, Hur J, Go DJ et al (2018) Baseline peripheral blood neutrophil-to-lymphocyte ratio could predict survival in patients with adult polymyositis and dermatomyositis: A retrospective observational study. PLoS ONE 13:e0190411. https://doi.org/10.1371/journal.pone.0190411
Medsger TA, Rodnan GP, Moossy J, Vester JW (1968) Skeletal muscle involvement in progressive systemic sclerosis (scleroderma). Arthritis Rheum 11:554–568. https://doi.org/10.1002/art.1780110405
Paik JJ, Wigley FM, Shah AA et al (2017) Fibrosing myopathy in systemic sclerosis associates with higher mortality. Arthritis Care Res 69:1764–1770. https://doi.org/10.1002/acr.23291
Fairley JL, Hansen D, Proudman S et al (2020) Clinical characteristics and survival in systemic sclerosis-mixed connective tissue disease and systemic sclerosis-overlap syndrome. Arthritis Care Res. https://doi.org/10.1002/acr.24167
Koschik RW, Fertig N, Lucas MR et al (2012) Anti-PM-Scl antibody in patients with systemic sclerosis. Clin Exp Rheumatol 30:S12-16
Liang Y, Leng RX, Pan HF, Ye DQ (2017) Associated variables of myositis in systemic lupus erythematosus: a cross-sectional study. Med Sci Monit Int Med J Exp Clin Res 23:2543–2549. Doi: https://doi.org/10.12659/MSM.902016
Garton MJ, Isenberg DA (1997) Clinical features of lupus myositis versus idiopathic myositis: a review of 30 cases. Rheumatology 36:1067–1074. https://doi.org/10.1093/rheumatology/36.10.1067
Jiang N, Li M, Zhang M et al (2019) Chinese SLE Treatment and Research group (CSTAR) registry: clinical significance of thrombocytopenia in Chinese patients with systemic lupus erythematosus. PLoS ONE 14:e0225516. https://doi.org/10.1371/journal.pone.0225516
Acknowledgments
None.
Funding
APLAR research grant 2017 awarded to LG.
Author information
Authors and Affiliations
Contributions
RN, UR, and LG collected and analyzed the clinical data. All authors were involved in writing and reviewing the manuscript for critical intellectual inputs.
Corresponding author
Ethics declarations
Conflicts of interest
R Naveen: none declared, Latika Gupta: none declared, Upendra Rathore: none declared, and Vikas Agarwal: none declared. The paper has been accepted as live oral presentation in APLAR 2020. Link: https://aplar.delegateconnect.co/talks/127
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Table 1.
Comorbidities, MSA/MAA and ANA of adults with IIM (n=214) (DOCX 16 KB)
Rights and permissions
About this article
Cite this article
Naveen, R., Rathore, U., Agarwal, V. et al. Characteristics and outcomes of overlap myositis: a comparative multigroup cohort study in adults from the MyoCite cohort. Rheumatol Int 41, 551–563 (2021). https://doi.org/10.1007/s00296-020-04779-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-020-04779-y