Abstract
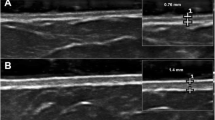
The objective is to detect any possible correlation between the modified Rodnan skin score (mRSS) and dermal thickness (DT) measured by skin high-frequency ultrasound (US) and the percentage of circulating fibrocytes in patients with limited cutaneous systemic sclerosis (lcSSc). Eight lcSSc patients and five healthy subjects (control group, CNT) were enrolled. The skin involvement was evaluated by mRSS and US (18 and 22 MHz probes) in all 13 subjects in the 17 standard skin areas evaluated by mRss. Circulating fibrocytes were isolated from the peripheral blood mononuclear cells (PBMCs) of all lcSSc patients and the CNT group to analyze their percentage at baseline time (T0) when the experiments started with PBMCs’ isolation and collection and after 8 days of culture (T8). Non-parametric tests were used for the statistical analysis. A positive correlation between the percentage of circulating fibrocytes at T0, mRSS (p = 0.04 r = 0.96), and DT-US, evaluated by the 22 MHz and the 18 MHz probes (p = 0.03, r = 0.66 and p = 0.05, r = 0.52, respectively), was observed in lcSSc patients. Conversely, at T8, there was no correlation (p > 0.05) between these parameters in lcSSc group. In the CNT group, no correlations between mRSS or DT-US and the percentage of circulating fibrocytes were observed both at T0 and T8. The study shows the presence of a significant relationship between the percentage of circulating fibrocytes and DT, as evidenced by both mRSS and US, in limited cutaneus SSc. This observation may well suggest the reasonable hypothesis of a crucial contribution of circulating fibrocytes to skin fibrosis progression, which might be considered as further biomarkers.

Similar content being viewed by others
References
Krieg T, Takehara K (2009) Skin disease: a cardinal feature of systemic sclerosis. Rheumatology 48:iii14–iii18
Cutolo M, Sulli A, Smith V (2010) Assessing microvascular changes in systemic sclerosis diagnosis and management. Nat Rev Rheumatol 6:578–587
van den Hoogen F, Khanna D, Fransen J et al (2013) 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 72:1747–1755
Clements P, Lachenbruch P, Siebold J et al (1995) Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol 22:1281–1285
Kaldas M, Khanna PP, Furst DE et al (2009) Sensitivity to change of the modified Rodnan skin score in diffuse systemic sclerosis—assessment of individual body sites in two large randomized controlled trials. Rheumatology 48:1143–1146
Czirják L, Nagy Z, Aringer M, Riemekasten G, Matucci-Cerinic M, Furst DE (2007) The EUSTAR model for teaching and implementing the modified Rodnan skin score in systemic sclerosis. Ann Rheum Dis 66:966–969
Kaloudi O, Bandinelli F, Filippucci E et al (2010) High frequency ultrasound measurement of digital dermal thickness in systemic sclerosis. Ann Rheum Dis 69:1140–1143
Moore TL, Lunt M, McManus B, Anderson ME, Herrick AL (2003) Seventeen-point dermal ultrasound scoring system-a reliable measure of skin thickness in patients with systemic sclerosis. Rheumatology 42:1559–1563
Sulli A, Ruaro B, Alessandri A et al (2014) Correlation between nailfold microangiopathy severity, finger dermal thickness and fingertip blood perfusion in systemic sclerosis patients. Ann Rheum Dis 73:247–251
Li H, Furst DE, Jin H et al (2018) High-frequency ultrasound of the skin in systemic sclerosis: an exploratory study to examine correlation with disease activity and to define the minimally detectable difference. Arthritis Res Ther. 20:181. https://doi.org/10.1186/s13075-018-1686-9
Ruaro B, Sulli A, Pizzorni C et al (2018) Correlations between blood perfusion and dermal thickness in different skin areas of systemic sclerosis patients. Microvasc Res 115:28–33
Santiago T, Santiago M, Ruaro B, Salvador MJ, Cutolo M, da Silva JAP (2018) Ultrasonography for the assessment of skin in systemic sclerosis: a systematic review. Arthritis Care Res (Hoboken). https://doi.org/10.1002/acr.23597 (Epub ahead of print)
Eyden B (2008) The myofibroblasts: phenotypic characterization as a prerequisite to understanding its functions in translational medicine. J Cell Mol Med 12:22–37
Galligan CL, Fish EN (2013) The role of circulating fibrocytes in inflammation and autoimmunity. J Leukoc Biol 93:45–50
Grieb G, Bucala R (2012) Fibrocytes in fibrotic diseases and wound healing. Adv Wound Care (New Rochelle). 1:36–40
Strieter RM, Keeley EC, Hughes MA, Burdick MD, Mehrad B (2009) The role of circulating mesenchymal progenitor cells (fibrocytes) in the pathogenesis of pulmonary fibrosis. J Leukoc Biol 86:1111–1118
Russo R, Medbury H, Guiffre A, Englert H, Manolios N (2007) Lack of increased expression of cell surface markers for circulating fibrocyte progenitors in limited scleroderma. Clin Rheumatol 26:1136–1141
Blakaj A, Bucala R (2012) Fibrocytes in health and disease. Fibrogenesis Tissue Repair 5(Suppl 1):S6. https://doi.org/10.1186/1755-1536-5-S1-S6
Cutolo M, Soldano S, Montagna P et al (2018) Effects of CTLA4-Ig treatment on circulating fibrocytes and skin fibroblasts from the same systemic sclerosis patients: an in vitro assay. Arthritis Res Ther 20:157. https://doi.org/10.1186/s13075-018-1652-6
Zeng J, Guo LW (2018) Signaling mechanisms of myofibroblast activation: outside-in and inside-out. Cell Physiol Biochem 49:848–868
Stempien-Otero A, Kim DH, Davis J (2016) Molecular networks underlying myofibroblast fate and fibrosis. J Mol Cell Cardiol 97:153–161
Micallef L, Vedrenne N, Billet F, Coulomb B, Darby I, Desmoulière A (2012) The myofibroblast, multiple origins for major roles in normal and pathological tissue repair. Fibrogenesis Tissue Repair 5(Suppl 1):S5
Bucala R (2015) Fibrocytes at 20 years. Mol Med 21(Suppl 1):S3–S5
Just SA, Lindegaard H, Hejbøl EK et al (2017) Fibrocyte measurement in peripheral blood correlates with number of cultured mature fibrocytes in vitro and is a potential biomarker for interstitial lung disease in rheumatoid arthritis. Respir Res 18:141
Abe R, Donnelly SC, Peng T, Bucala R, Metz CN (2001) Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 166:7556–7562
Mori L, Bellini A, Stacey MA, Schmidt M, Mattoli S (2005) Fibrocytes contribute to the myofibroblast population in the wounded skin and originate from the bone marrow. Exp Cell Res 304:81–90
Liu Y, Qingjuan S, Gao Z, Deng C, Wang Y, Guo C (2017) Circulating fibrocytes are involved in inflammation and leukocyte trafficking in neonates with necrotizing enterocolitis. Medicine (Baltimore) 96:e7400. https://doi.org/10.1097/MD.0000000000007400
Dupin I, Allard B, Ozier A et al (2016) Blood fibrocytes are recruited during acute exacerbations of chronic obstructive pulmonary disease through a CXCR28-dependent pathway. J Allergy Clin Immunol 137:1036–1042
Cutolo M, Ruaro B, Montagna P et al (2018) Effects of selexipag and its active metabolite in contrasting the profibrotic myofibroblast activity in cultured scleroderma skin fibroblasts. Arthritis Res Therapy 20:77
Sulli A, Soldano S, Pizzorni C et al (2009) Raynaud’s phenomenon and plasma endothelin: correlations with capillaroscopic patterns in systemic sclerosis. J Rheumatol 36:1235–1239
Dupin I, Allard B, Ozier A et al (2016) Blood fibrocytes are recruited during acute exacerbations of chronic obstructive pulmonary disease through a CXCR31-dependent pathway. J Allergy Clin Immunol 137:1036–1042
Cutolo M, Sulli A, Pizzorni C, Accardo S (2000) Nailfold videocapillaroscopy assessment of microvascular damage in systemic sclerosis. J Rheumatol 27:155–160
Sulli A, Secchi ME, Pizzorni C, Cutolo M (2008) Scoring the nailfold microvascular changes during the capillaroscopic analysis in systemic sclerosis patients. Ann Rheum Dis 67:885–887
Pizzorni C, Sulli A, Smith V et al (2017) Primary Raynaud’s phenomenon and nailfold videocapillaroscopy: age-related changes in capillary morphology. Clin Rheumatol 36:1637–1642
Ruaro B, Casabella A, Paolino S et al (2018) Dickkopf-1 (Dkk-1) serum levels in systemic sclerosis and rheumatoid arthritis patients: correlation with the Trabecular Bone Score (TBS). Clin Rheumatol 37:3057–3062
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C (T)) method. Methods 25:402–408
Brunasso AM, Massone C (2016) Update on the pathogenesis of Scleroderma: focus on circulating progenitor cells. F1000 Res. https://doi.org/10.12688/f1000research.7986.1 (F1000 Faculty Rev-723. eCollection 2016)
Reilkoff R, Bucal R, Herzog E (2011) Fibrocytes: emerging effector cells in chronic inflammation. Nat Rev Immunol 11:427–435
Sun M, Wang P, Okubo T et al (2018) Possible contribution of fibrocytes to increased type i collagen synthesis during the early stage of dermal wound repair in human skin. J Invest Dermatol 138:240–242
Bagnato G, Harari S (2015) Cellular interactions in the pathogenesis of interstitial lung diseases. Eur Respir Rev 24:102–114
Moore BB, Kolodsick JE, Thannickal VJ et al (2005) CCR41-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol 166:675–684
Strieter RM, Keeley EC, Hughes MA, Burdick MD, Mehrad B (2009) The role of circulating mesenchymal progenitor cells (fibrocytes) in the pathogenesys of pulmonary fibrosis. J Leukoc Biol 86:1111–1118
Keeley EC, Mehrad B, Strieter RM (2009) The role of circulating mesenchymal progenitor cells (fibrocytes) in the pathogenesis of fibrotic disorders. Thromb Haemost 101:613–618
Phillips RJ, Burdick MD, Hong K et al (2004) Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest 114:438–446
Bucala R (2008) Circulating fibrocytes: cellular basis for NSF. J Am Coll Radiol 5:36–39
Chu PY, Mariani J, Finch S et al (2010) Bone marrow-derived cells contribute to fibrosis in the chronically failing heart. Am J Pathol 176:1735–1742
Acknowledgements
The authors would like to thank Barbara Wade, contract Professor at the University of Torino, for her linguistic advice. Professor Vanessa Smith is a Senior Clinical Investigator of the Research Foundation—Flanders (Belgium) (FWO) [1802915 N]. Barbara Ruaro was supported by an EULAR scientific training bursary.
Funding
No specific funding was received from any bodies in the public, commercial or nonprofit sectors to carry out the research reported in this manuscript.
Author information
Authors and Affiliations
Contributions
All authors have: (1) made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; (2) been involved in drafting the manuscript or revising it critically for important intellectual content; (3) given final approval of the version to be published; and (4) agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests with this work.
Ethical approval
Study was approved by Ethics Committee of the IRCCS San Martino Polyclinic Hospital of Genoa (Protocol Number 273-REG-2015).
Research involving human participants
This article contains a study with human participants.
Informed consent
The patients were enrolled after having obtained their written informed consent for the use of imaging and the demographic data as educational material and for publications. This study was carried out in accordance with the ethical standards stipulated in the 1964 Declaration of Helsinki and its later amendments and was evaluated by the local IRB.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ruaro, B., Soldano, S., Smith, V. et al. Correlation between circulating fibrocytes and dermal thickness in limited cutaneous systemic sclerosis patients: a pilot study. Rheumatol Int 39, 1369–1376 (2019). https://doi.org/10.1007/s00296-019-04315-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-019-04315-7