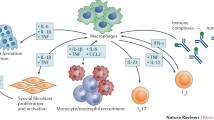
Abstract
As a component of the innate immune system, macrophages play a crucial role in host defense against a variety of microbes. Conventionally, macrophages have been classified as M1 and M2 depending on their phenotype and role in immune regulation. M1 macrophages are generally pro-inflammatory, while M2 (also known as alternatively activated macrophages) are anti-inflammatory. M1 macrophages release pro-inflammatory cytokines, reactive nitrogen, and oxygen intermediates, and kill pathogens, whereas their M2 counterparts participate in the resolution of inflammation, remodeling of tissue, angiogenesis, and tissue repair. Macrophages are also crucial in the pathogenesis of immune-inflammatory disorders, such as, arthritis. In this review, we discuss the markers of human M2 macrophages, the role played by them in inflammation or progression of rheumatic diseases, their potential to act as biomarkers, and, finally, therapeutic strategies aiming at altering/enhancing the macrophage phenotype.


Similar content being viewed by others
References
Metchnikoff E (1989) On the present state of the question of immunity in infectious diseases. Scand J Immunol 4:387–398
Mackaness GB (1962) Cellular resistance to infection. J Exp Med 116:381–406
Epelman S, Lavine KJ, Randolph GJ (2014) Origin and functions of tissue macrophages. Immunity 41(1):21–35
Stein M, Keshav S, Harris N, Gordon S (1992) Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med 176(1):287–292
Mills CD, Ley K (2014) M1 and M2 macrophages: the chicken and the egg of immunity. J Innate Immun 6(6):716–726
Roszer T (2015) Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediat Inflamm 2015:816480
El Kasmi KC, Qualls JE, Pesce JT et al (2008) Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat Immunol 9(12):1399–1406
Yang Z, Ming XF (2014) Functions of arginase isoforms in macrophage inflammatory responses: impact on cardiovascular diseases and metabolic disorders. Front Immunol 5:533
Porcheray F, Viaud S, Rimaniol A-C et al (2005) Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol 142(3):481–489
Svensson-Arvelund J, Mehta RB, Lindau R et al (2015) The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory T cells and homeostatic M2 macrophages. J Immunol 194(4):1534–1544
Dupasquier M, Stoitzner P, Wan H et al (2006) The dermal microenvironment induces the expression of the alternative activation marker CD301/mMGL in mononuclear phagocytes, independent of IL-4/IL-13 signaling. J Leukoc Biol 80(4):838–849
Zeyda M, Farmer D, Todoric J et al (2007) Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond) 31(9):1420–1428
Titos E, Rius B, González-Périz A et al (2011) Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J Immunol 187(10):5408–5418
Nawaz A, Aminuddin A, Kado T et al (2017) CD206+ M2-like macrophages regulate systemic glucose metabolism by inhibiting proliferation of adipocyte progenitors. Nat Commun 8(1):286
Madsen DH, Leonard D, Masedunskas A et al (2013) M2-like macrophages are responsible for collagen degradation through a mannose receptor-mediated pathway. J Cell Biol 202(6):951–966
Gao S, Zhou J, Liu N et al (2015) Curcumin induces M2 macrophage polarization by secretion IL-4 and/or IL-13. J Mol Cell Cardiol 85:131–139
Fabriek BO, Dijkstra CD, van den Berg TK (2005) The macrophage scavenger receptor CD163. Immunobiology 210(2–4):153–160
Law SK, Micklem KJ, Shaw JM, Zhang XP, Dong Y, Willis AC, Mason DY (1993) A new macrophage differentiation antigen which is a member of the scavenger receptor superfamily. Eur J Immunol 23(9):2320–2325
Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK (2001) Identification of the hemoglobin scavenger receptor. Nature 409(6817):198–201
Fabriek BO, Polfliet MM, Vloet RP et al (2007) The macrophage CD163 surface glycoprotein is an erythroblast adhesion receptor. Blood 109(12):5223–5229
Fabriek BO, van Bruggen R, Deng DM et al (2009) The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood 113(4):887–892
Loures FV, Araújo EF, Feriotti C, Bazan SB, Costa TA, Brown GD, Calich VL (2014) Dectin-1 induces M1 macrophages and prominent expansion of CD8+IL-17+ cells in pulmonary Paracoccidioidomycosis. J Infect Dis 210(5):762–773
Feriotti C, Bazan SB, Loures FV, Araújo EF, Costa TA, Calich VL (2015) Expression of dectin-1 and enhanced activation of NALP3 inflammasome are associated with resistance to paracoccidioidomycosis. Front Microbiol 6:913
Gales A, Conduche A, Bernad J, Lefevre L, Olagnier D, Beraud M, Martin-Blondel G (2010) PPARgamma controls Dectin-1 expression required for host antifungal defense against Candida albicans. PLoS Pathog 6(1):e1000714
Nandakumar V, Hebrink D, Jenson P, Kottom T, Limper AH (2017) Differential macrophage polarization from pneumocystis in immunocompetent and immunosuppressed hosts: potential adjunctive therapy during pneumonia. Infect Immun 85(3):e00939–e00916
Willment JA, Lin HH, Reid DM et al (2003) Dectin-1 expression and function are enhanced on alternatively activated and GM-CSF-treated macrophages and are negatively regulated by IL-10, dexamethasone, and lipopolysaccharide. J Immunol 171(9):4569–4573
Zizzari IG, Napoletano C, Battisti F et al (2015) MGL receptor and immunity: when the ligand can make the difference. J Immunol Res 2015:450695
Wang Y, Han CC, Cui D, Li Y, Ma Y, Wei W (2017) Is macrophage polarization important in rheumatoid arthritis? Int Immunopharmacol 50:345–352
Zhao J, Yuan W, Tao C, Sun P, Yang Z, Xu W (2017) M2 polarization of monocytes in ankylosing spondylitis and relationship with inflammation and structural damage. APMIS 125(12):1070–1075
Ciccia F, Alessandro R, Rizzo A et al (2014) Macrophage phenotype in the subclinical gut inflammation of patients with ankylosing spondylitis. Rheumatology 53(1):104–113
Ambarus CA, Noordenbos T, de Hair MJ, Tak PP, Baeten DL (2012) Intimal lining layer macrophages but not synovial sublining macrophages display an IL-10 polarized-like phenotype in chronic synovitis. Arthritis Res Ther 14(2):R74
Vandooren B, Noordenbos T, Ambarus C et al (2009) Absence of a classically activated macrophage cytokine signature in peripheral spondylarthritis, including psoriatic arthritis. Arthritis Rheum 60(4):966–975
Fuentes-Duculan J, Suárez-Fariñas M, Zaba LC et al (2010) A subpopulation of CD163-positive macrophages is classically activated in psoriasis. J Investig Dermatol 130(10):2412–2422
Seo DH, Che X, Kwak MS et al (2017) Interleukin-33 regulates intestinal inflammation by modulating macrophages in inflammatory bowel disease. Sci Rep 7(1):851
Zhu W, Yu J, Nie Y, Shi X, Liu Y, Li F, Zhang XL (2014) Disequilibrium of M1 and M2 macrophages correlates with the development of experimental inflammatory bowel diseases. Immunol Investig 43(7):638–652
Salvador P, Macías-Ceja DC, Gisbert-Ferrándiz L et al (2018) CD16+ macrophages mediate fibrosis in inflammatory bowel disease. J Crohns Colitis 12(5):589–599
Janka GE (2012) Familial and acquired hemophagocytic lymphohistiocytosis. Annu Rev Med 63:233–246
Bleesing J, Prada A, Siegel DM et al (2007) The diagnostic significance of soluble CD163 and soluble interleukin-2 receptor alpha-chain in macrophage activation syndrome and untreated new-onset systemic juvenile idiopathic arthritis. Arthritis Rheum 56(3):965–971
Møller HJ, Aerts H, Grønbaek H et al (2002) Soluble CD163: a marker molecule for monocyte/macrophage activity in disease. Scand J Clin Lab Investig Suppl 237:29–33
Fall N, Barnes M, Thornton S et al (2007) Gene expression profiling of peripheral blood from patients with untreated new-onset systemic juvenile idiopathic arthritis reveals molecular heterogeneity that may predict macrophage activation syndrome. Arthritis Rheum 56(11):3793–3804
Sakumura N, Shimizu M, Mizuta M, Inoue N, Nakagishi Y, Yachie A (2018) Soluble CD163, a unique biomarker to evaluate the disease activity, exhibits macrophage activation in systemic juvenile idiopathic arthritis. Cytokine 18:30216-3
Reddy VV, Myles A, Cheekatla SS, Singh S, Aggarwal A (2014) Soluble CD25 in serum: a potential marker for subclinical macrophage activation syndrome in patients with active systemic onset juvenile idiopathic arthritis. Int J Rheum Dis 17(3):261–267
Schaer CA, Vallelian F, Imhof A, Schoedon G, Schaer DJ (2008) Heme carrier protein (HCP-1) spatially interacts with the CD163 hemoglobin uptake pathway and is a target of inflammatory macrophage activation. J Leukoc Biol 83(2):325–333
Aggarwal A, Gaur P, Yadav A (2017) Evidence for alternatively activated (M2) macrophage activation in patients with enthesitis related arthritis category of juvenile idiopathic arthritis. In: ACR/ARHP annual meeting, San Diego
Fukui S, Iwamoto N, Takatani A, Igawa T, Shimizu T, Umeda M, Nishino A (2017) M1 and M2 Monocytes in Rheumatoid Arthritis: A Contribution of Imbalance of M1/M2 Monocytes to Osteoclastogenesis. Front Immunol 8:1958
Mottonen M, Isomaki P, Saario R, Toivanen P, Punnonen J, Lassila O (1998) Interleukin-10 inhibits the capacity of synovial macrophages to function as antigen-presenting cells. Br J Rheumatol 37(11):1207–1214
Zhu W, Li X, Fang S, Zhang X, Wang Y, Zhang T, Li Z (2015) Anti-citrullinated protein antibodies induce macrophage subset disequilibrium in RA patients. Inflammation 38(6):2067–2075
Vogelpoel LT, Hansen IS, Rispens T, Muller FJ, van Capel TM, Turina MC, Vos JB (2014) Fc gamma receptor-TLR cross-talk elicits pro-inflammatory cytokine production by human M2 macrophages. Nat Commun 5:5444
Sun W, Zhang H, Wang H, Chiu YG, Wang M, Ritchlin CT, Kiernan A (2017) Targeting notch-activated M1 macrophages attenuates joint tissue damage in a mouse model of inflammatory arthritis. J Bone Miner Res 32(7):1469–1480
Narayan N, Owen DR, Mandhair H, Smyth E, Carlucci F, Saleem A, Gunn RN (2018) Translocator protein as an imaging marker of macrophage and stromal activation in rheumatoid arthritis pannus. J Nucl Med 59(7):1125–1132
Daghestani HN, Pieper CF, Kraus VB (2015) Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis Rheumatol 67(4):956–965
van Lent PL, Blom AB, van der Kraan P, Holthuysen AE, Vitters E, van Rooijen N, Smeets RL (2004) Crucial role of synovial lining macrophages in the promotion of transforming growth factor beta-mediated osteophyte formation. Arthritis Rheum 50(1):103–111
Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B (2005) Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis 64(9):1263–1267
Utomo L, Bastiaansen-Jenniskens YM, Verhaar JA, van Osch GJ (2016) Cartilage inflammation and degeneration is enhanced by pro-inflammatory (M1) macrophages in vitro, but not inhibited directly by anti-inflammatory (M2) macrophages. Osteoarthr Cartil 24(12):2162–2170
Fahy N, de Vries-van Melle ML, Lehmann J, Wei W, Grotenhuis N, Farrell E (2014) Human osteoarthritic synovium impacts chondrogenic differentiation of mesenchymal stem cells via macrophage polarisation state. Osteoarthr Cartil 22(8):1167–1175
Jeong JH, Hong S, Kwon OC, Ghang B, Hwang I, Kim YG, Lee CK (2017) CD14(+) Cells with the Phenotype of Infiltrated Monocytes Consist of Distinct Populations Characterized by Anti-inflammatory as well as Pro-inflammatory Activity in Gouty Arthritis. Front Immunol 8:1260
Martin WJ, Walton M, Harper J (2009) Resident macrophages initiating and driving inflammation in a monosodium urate monohydrate crystal-induced murine peritoneal model of acute gout. Arthritis Rheum 60(1):281–289
Garcia-Melchor E, Yagüe J, Juan M, Harper J (2014) Macrophages-Mediated Response to Uric Acid Crystals Is Modulated By Their Functional Polarization. Arthritis Rheum 66(10,suppl):95959
Li J, Yu YF, Liu CH, Wang CM (2017) Significance of M2 macrophages in glomerulonephritis with crescents. Pathol Res Pract 213(9):1215–1220
Li J, Liu CH, Gao B, Xu DL (2015) Clinical-pathologic significance of CD163 positive macrophage in IgA nephropathy patients with crescents. Int J Clin Exp Med 8(6):9299–9305
Zhao L, David MZ, Hyjek E, Chang A, Meehan SM (2015) M2 macrophage infiltrates in the early stages of ANCA-associated pauci-immune necrotizing GN. Clin J Am Soc Nephrol 10(1):54–62
Han S, Zhuang H, Shumyak S, Wu J, Li H, Yang LJ, Reeves WH (2017) A novel subset of anti-inflammatory CD138+ macrophages is deficient in mice with experimental lupus. J Immunol 199(4):1261–1274
Cai Y, Zhang W, Xion S (2013) Mannose-binding lectin blunts macrophage polarization and ameliorates lupus nephritis. PLoS One 8(4):e62465
Nakayama W, Jinnin M, Makino K et al (2012) CD163 expression is increased in the involved skin and sera of patients with systemic lupus erythematosus. Eur J Dermatol 22(4):512–517
Zizzo G, Guerrieri J, Dittman LM, Merrill JT, Cohen PL (2013) Circulating levels of soluble MER in lupus reflect M2c activation of monocytes/macrophages, autoantibody specificities and disease activity. Arthritis Res Ther 15(6):R212
Olmes G, Büttner-Herold M, Ferrazzi F, Distel L, Amann K, Daniel C (2016) CD163+ M2c-like macrophages predominate in renal biopsies from patients with lupus nephritis. Arthritis Res Ther 18:90
Endo N, Tsuboi N, Furuhashi K et al (2016) Urinary soluble CD163 level reflects glomerular inflammation in human lupus nephritis. Nephrol Dial Transplant 31(12):2023–2033
Gupta R, Yadav A, Aggarwal A (2016) Urinary soluble CD163, an M2 macrophage marker, reflects the renal disease activity in lupus nephritis: a cross sectional and longitudinal assessment. Arthritis Rheumatol 68(10 Suppl):1282
York MR, Nagai T, Mangini AJ, Lemaire R, van Seventer JM, Lafyatis R (2007) A macrophage marker, Siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by type I interferons and toll-like receptor agonists. Arthritis Rheum 56(3):1010–1020
Higashi-Kuwata N, Jinnin M, Makino T et al (2010) Characterization of monocyte/macrophage subsets in the skin and peripheral blood derived from patients with systemic sclerosis. Arthritis Res Ther 12(4):R128
Wynn TA, Vannella KM (2016) Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44(3):450–462
Bielecki M, Kowal K, Lapinska A, Chyczewski L, Kowal-Bielecka O (2013) Increased release of soluble CD163 by the peripheral blood mononuclear cells is associated with worse prognosis in patients with systemic sclerosis. Adv Med Sci 58(1):126–133
Mathai SK, Gulati M, Peng X, Russell TR, Shaw AC, Rubinowitz AN, Murray LA (2010) Circulating monocytes from systemic sclerosis patients with interstitial lung disease show an enhanced profibrotic phenotype. Lab Investig 90(6):812–823
Christmann RB, Hayes E, Pendergrass S, Padilla C, Farina G, Affandi AJ, Whitfield ML (2011) Interferon and alternative activation of monocyte/macrophages in systemic sclerosis-associated pulmonary arterial hypertension. Arthritis Rheum 63(6):1718–1728
Maier C, Ramming A, Bergmann C, Weinkam R, Kittan N, Schett G, Distler JHW (2017) Inhibition of phosphodiesterase 4 (PDE4) reduces dermal fibrosis by interfering with the release of interleukin-6 from M2 macrophages. Ann Rheum Dis 76(6):1133–1141
Furukawa S, Moriyama M, Miyake K, Nakashima H, Tanaka A, Maehara T, Iizuka-Koga M (2017) Interleukin-33 produced by M2 macrophages and other immune cells contributes to Th2 immune reaction of IgG4-related disease. Sci Rep 7:42413
Furukawa S, Moriyama M, Tanaka A, Maehara T, Tsuboi H, Iizuka M, Hayashida JN (2015) Preferential M2 macrophages contribute to fibrosis in IgG4-related dacryoadenitis and sialoadenitis, so-called Mikulicz’s disease. Clin Immunol 156(1):9–18
Kodelja V, Muller C, Politz O, Hakij N, Orfanos CE, Goerdt S (1998) Alternative macrophage activation-associated CC-chemokine-1, a novel structural homologue of macrophage inflammatory protein-1 alpha with a Th2-associated expression pattern. J Immunol 160(3):1411–1418
Akiyama M, Yasuoka H, Yoshimoto K, Takeuchi T (2017) CC-chemokine ligand 18 is a useful biomarker associated with disease activity in IgG4-related disease. Ann Rheum Dis 2017:212110
Aota K, Yamanoi T, Kani K, Nakashiro KI, Ishimaru N, Azuma M (2018) Inverse correlation between the number of CXCR3(+) macrophages and the severity of inflammatory lesions in Sjogren’s syndrome salivary glands: a pilot study. J Oral Pathol Med. https://doi.org/10.1111/jop.12756
Caorsi R, Penco F, Schena F, Gattorno M (2016) Monogenic polyarteritis: the lesson of ADA2 deficiency. Pediatr Rheumatol Online J 14(1):51
Zhou Q, Yang D, Ombrello AK, Zavialov AV, Toro C, Zavialov AV, Stone DL (2014) Early-onset stroke and vasculopathy associated with mutations in ADA2. N Engl J Med 370(10):911–920
Alvarado-Vazquez PA, Bernal L, Paige CA et al (2017) Macrophage-specific nanotechnology-driven CD163 overexpression in human macrophages results in an M2 phenotype under inflammatory conditions. Immunobiology 222(8–9):900–912
Hayder M, Poupot M, Baron M, Nigon D, Turrin CO, Caminade AM, Majoral JP (2011) A phosphorus-based dendrimer targets inflammation and osteoclastogenesis in experimental arthritis. Sci Transl Med 3(81):81ra35
Mills EL, Kelly B, Logan A, Costa ASH, Varma M, Bryant C, Tourlomousis P (2016) Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 167(2):457–470
Zhang J, Lin Y, Li C et al (2016) IL-35 decelerates the inflammatory process by regulating inflammatory cytokine secretion and M1/M2 macrophage ratio in psoriasis. J Immunol 197(6):2131–2144
Shin TH, Kim HS, Kang TW et al (2016) Human umbilical cord blood-stem cells direct macrophage polarization and block inflammasome activation to alleviate rheumatoid arthritis. Cell Death Dis 7(12):e2524
Jain S, Tran TH, Amiji M (2015) Macrophage repolarization with targeted alginate nanoparticles containing IL-10 plasmid DNA for the treatment of experimental arthritis. Biomaterials 61:162–177
Li J, Hsu HC, Ding Y, Li H, Wu Q, Yang P, Luo B (2014) Inhibition of fucosylation reshapes inflammatory macrophages and suppresses type II collagen-induced arthritis. Arthritis Rheumatol 66(9):2368–2379
Sultana F, Neog MK, Rasool M (2017) Withaferin-A, a steroidal lactone encapsulated mannose decorated liposomes ameliorates rheumatoid arthritis by intriguing the macrophage repolarization in adjuvant-induced arthritic rats. Colloids Surf B Biointerfaces 155:349–365
Tong WW, Zhang C, Hong T, Liu DH, Wang C, Li J, He XK (2018) Silibinin alleviates inflammation and induces apoptosis in human rheumatoid arthritis fibroblast-like synoviocytes and has a therapeutic effect on arthritis in rats. Sci Rep 8(1):3241
Siebelt M, Korthagen N, Wei W, Groen H, Bastiaansen-Jenniskens Y, Muller C, Waarsing JH (2015) Triamcinolone acetonide activates an anti-inflammatory and folate receptor-positive macrophage that prevents osteophytosis in vivo. Arthritis Res Ther 17:352
Dhanasekar C, Kalaisevan S, Rasool M (2015) Morin, a bioflavonoid suppresses monosodium urate crystal-induced inflammatory immune response in RAW 264.7 macrophages through the inhibition of inflammatory mediators, intracellular ROS levels and NF-κB activation. PLoS One 10(12):e0145093
Lee SY, Lee SW, Lee SY, Hong KW, Bae SS, Kim K, Kim CD (2017) SIRT1/adenosine monophosphate-activated protein kinase alpha signaling enhances macrophage polarization to an anti-inflammatory phenotype in rheumatoid arthritis. Front Immunol 8:1135
Furuhashi K, Tsuboi N, Shimizu A, Katsuno T, Kim H, Saka Y, Ozaki T (2013) Serum-starved adipose-derived stromal cells ameliorate crescentic GN by promoting immunoregulatory macrophages. J Am Soc Nephrol 24(4):587–603
Li F, Yang Y, Zhu X, Huang L, Xu J (2015) Macrophage polarization modulates development of systemic lupus erythematosus. Cell Physiol Biochem 37(4):1279–1288
Liu R, Fan T, Geng W, Chen Y, Ruan Q, Zhang C (2017) Negative immune regulator TIPE2 promotes M2 macrophage differentiation through the activation of PI3K-AKT signaling pathway. PLoS One 12(1):e0170666
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhattacharya, S., Aggarwal, A. M2 macrophages and their role in rheumatic diseases. Rheumatol Int 39, 769–780 (2019). https://doi.org/10.1007/s00296-018-4120-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-018-4120-3