Abstract
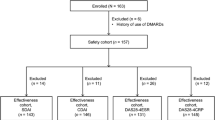
The utility of monitoring drug levels in rheumatoid arthritis and spondyloarthritis patients on biological therapy is called into question. The objective was to study relevant clinical questions on the topic, i.e., (1) whether drug levels predict relapse in patients whose biologic was optimized because of remission or low disease activity; (2) whether information about drug levels influences the prognosis of patients with primary or secondary failure to a biological therapy; and (3) whether methotrexate (MTX) influences the association between drug levels and response. Medline, Embase, Cochrane databases were screened, from inception to December 2016 in search for all studies related to the three research questions about. Overall characteristics and outcomes of the studies were collected in a table of evidence and the quality of the studies was assessed with the Newcastle-Ottawa scale or the GRADEpro. Two studies responded the first question, 5 the second, and 7 the third. Studies were small and with limitations, but suggest that measurement drug levels may be useful in patients in remission; that higher drug levels predict a longer relapse-free optimization, and in patients with failure to a biological agent, treatment may need individual adjustment according to the presence of drug levels or antidrug-antibodies. In addition, MTX influences the association between response and drug levels. Monitoring drug levels would allow optimal use of current biological therapies, but more studies and of better quality are needed to draw definitive conclusions.

Similar content being viewed by others
References
Nam JL, Ramiro S, Gaujoux-Viala C, Takase K et al (2014) Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2013 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 73(3):516–528. https://doi.org/10.1136/annrheumdis-2013-204577
Sepriano A, Regel A, van der Heijde D, Braun J et al (2017) Efficacy and safety of biological and targeted-synthetic DMARDs: a systematic literature review informing the 2016 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. RMD Open 3(1):e000396. https://doi.org/10.1136/rmdopen-2016-000396
Corbett M, Soares M, Jhuti G, Rice S et al (2016) Tumour necrosis factor-alpha inhibitors for ankylosing spondylitis and non-radiographic axial spondyloarthritis: a systematic review and economic evaluation. Health Technol Assess 20(9):1-334, v-vi. https://doi.org/10.3310/hta20090
Kuijper TM, Buisman LR, Hazes JM, Weel AE (2017) Cost-effectiveness of biological disease-modifying antirheumatic drugs for the treatment of rheumatoid arthritis: implications for clinical practice. J Rheumatol 44(7):965–967. https://doi.org/10.3899/jrheum.170334
Takeuchi T, Miyasaka N, Inoue K, Abe T et al (2009) Impact of trough serum level on radiographic and clinical response to infliximab plus methotrexate in patients with rheumatoid arthritis: results from the RISING study. Mod Rheumatol 19(5):478–487. https://doi.org/10.1007/s10165-009-0195-8
St Clair EW, Wagner CL, Fasanmade AA, Wang B et al (2002) The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 46(6):1451–1459. https://doi.org/10.1002/art.10302
Chen DY, Chen YM, Tsai WC, Tseng JC et al (2015) Significant associations of antidrug antibody levels with serum drug trough levels and therapeutic response of adalimumab and etanercept treatment in rheumatoid arthritis. Ann Rheum Dis 74(3):e16. https://doi.org/10.1136/annrheumdis-2013-203893
Paramarta JE, Baeten DL (2014) Adalimumab serum levels and antidrug antibodies towards adalimumab in peripheral spondyloarthritis: no association with clinical response to treatment or with disease relapse upon treatment discontinuation. Arthritis Res Ther 16(4):R160. https://doi.org/10.1186/ar4675
Kneepkens EL, Krieckaert CL, van der Kleij D, Nurmohamed MT et al (2015) Lower etanercept levels are associated with high disease activity in ankylosing spondylitis patients at 24 weeks of follow-up. Ann Rheum Dis 74(10):1825–1829. https://doi.org/10.1136/annrheumdis-2014-205213
Jani M, Isaacs JD, Morgan AW, Wilson AG et al (2017) High frequency of antidrug antibodies and association of random drug levels with efficacy in certolizumab pegol-treated patients with rheumatoid arthritis: results from the BRAGGSS cohort. Ann Rheum Dis 76(1):208–213. https://doi.org/10.1136/annrheumdis-2015-208849
Kneepkens EL, Plasencia C, Krieckaert CL, Pascual-Salcedo D et al (2014) Golimumab trough levels, antidrug antibodies and clinical response in patients with rheumatoid arthritis treated in daily clinical practice. Ann Rheum Dis 73(12):2217–2219. https://doi.org/10.1136/annrheumdis-2014-205983
Benucci M, Meacci F, Grossi V, Infantino M et al (2016) Correlations between immunogenicity, drug levels, and disease activity in an Italian cohort of rheumatoid arthritis patients treated with tocilizumab. Biologics 10:53–58. https://doi.org/10.2147/BTT.S97234
Mazilu D, Opris D, Gainaru C, Iliuta M et al. (2014) Monitoring drug and antidrug levels: a rational approach in rheumatoid arthritis patients treated with biologic agents who experience inadequate response while being on a stable biologic treatment. Biomed Res Int 2014:702701. https://doi.org/10.1155/2014/702701
Wells G, Shea B, O’Connel D (2009 Feb 1) The Newcastle-Ottawa scale (NOS) for assessing the quailty of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed 03 Mar 2018
Schünemann H, Brozek J, Guyatt G, Oxman A (2013) GRADE handbook for grading quality of evidence and strength of recommendations [Internet]. The GRADE Working Group. http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html. Accessed 03 Mar 2018
Chen DY, Chen YM, Hsieh TY, Hung WT et al (2016) Drug trough levels predict therapeutic responses to dose reduction of adalimumab for rheumatoid arthritis patients during 24 weeks of follow-up. Rheumatology 55(1):143–148. https://doi.org/10.1093/rheumatology/kev298
Meric JC, Mulleman D, Ducourau E, Lauferon F et al (2011) Therapeutic drug monitoring of infliximab in spondyloarthritis: an observational open-label study. Ther Drug Monit 33(4):411–416. https://doi.org/10.1097/FTD.0b013e318224f83d
Mulleman D, Meric JC, Paintaud G, Ducourau E et al (2009) Infliximab concentration monitoring improves the control of disease activity in rheumatoid arthritis. Arthritis Res Ther 11(6):R178. https://doi.org/10.1186/ar2867
Garces S, Antunes M, Benito-Garcia E, da Silva JC et al (2014) A preliminary algorithm introducing immunogenicity assessment in the management of patients with RA receiving tumour necrosis factor inhibitor therapies. Ann Rheum Dis 73(6):1138–1143. https://doi.org/10.1136/annrheumdis-2013-203296
Plasencia C, Jurado T, Villalba A, Peitedado D et al (2015) Effect of infliximab dose increase in rheumatoid arthritis at different trough concentrations: a cohort study in clinical practice conditions. Front Med (Lausanne) 2:71. https://doi.org/10.3389/fmed.2015.00071
Jamnitski A, Krieckaert CL, Nurmohamed MT, Hart MH et al (2012) Patients non-responding to etanercept obtain lower etanercept concentrations compared with responding patients. Ann Rheum Dis 71(1):88–91. https://doi.org/10.1136/annrheumdis-2011-200184
Burmester GR, Kivitz AJ, Kupper H, Arulmani U et al (2015) Efficacy and safety of ascending methotrexate dose in combination with adalimumab: the randomised CONCERTO trial. Ann Rheum Dis 74(6):1037–1044. https://doi.org/10.1136/annrheumdis-2013-204769
Pouw MF, Krieckaert CL, Nurmohamed MT, van der Kleij D et al (2015) Key findings towards optimising adalimumab treatment: the concentration-effect curve. Ann Rheum Dis 74(3):513–518. https://doi.org/10.1136/annrheumdis-2013-204172
Eng GP, Bouchelouche P, Bartels EM, Bliddal H et al (2016) Anti-drug antibodies, drug levels, interleukin-6 and soluble TNF receptors in rheumatoid arthritis patients during the first 6 months of treatment with adalimumab or infliximab: a descriptive cohort study. PLoS One 11(9):e0162316. https://doi.org/10.1371/journal.pone.0162316
Sigaux J, Hamze M, Daien C, Morel J et al (2017) Immunogenicity of tocilizumab in patients with rheumatoid arthritis. Joint Bone Spine 84(1):39–45. https://doi.org/10.1016/j.jbspin.2016.04.013
Krzysiek R, Breban M, Ravaud P, Prejean MV et al (2009) Circulating concentration of infliximab and response to treatment in ankylosing spondylitis: results from a randomized control study. Arthritis Rheum 61(5):569–576. https://doi.org/10.1002/art.24275
Ducourau E, Mulleman D, Paintaud G, Miow Lin DC et al (2011) Antibodies toward infliximab are associated with low infliximab concentration at treatment initiation and poor infliximab maintenance in rheumatic diseases. Arthritis Res Ther 13(3):R105. https://doi.org/10.1186/ar3386
Bartelds GM, Krieckaert CL, Nurmohamed MT, van Schouwenburg PA et al (2011) Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA 305(14):1460–1468. https://doi.org/10.1001/jama.2011.406
Krieckaert CL, Nair SC, Nurmohamed MT, van Dongen CJ et al (2015) Personalised treatment using serum drug levels of adalimumab in patients with rheumatoid arthritis: an evaluation of costs and effects. Ann Rheum Dis 74(2):361–368. https://doi.org/10.1136/annrheumdis-2013-204101
Rosas J, Llinares-Tello F, de la Torre I, Santos-Ramirez C et al (2014) Clinical relevance of monitoring serum levels of adalimumab in patients with rheumatoid arthritis in daily practice. Clin Exp Rheumatol 32(6):942–948
Bendtzen K, Geborek P, Svenson M, Larsson L et al (2006) Individualized monitoring of drug bioavailability and immunogenicity in rheumatoid arthritis patients treated with the tumor necrosis factor alpha inhibitor infliximab. Arthritis Rheum 54(12):3782–3789. https://doi.org/10.1002/art.22214
Strand V, Balsa A, Al-Saleh J, Barile-Fabris L et al (2017) Immunogenicity of biologics in chronic inflammatory diseases: a systematic review. BioDrugs. https://doi.org/10.1007/s40259-017-0231-8
Wolbink GJ, Vis M, Lems W, Voskuyl AE et al (2006) Development of antiinfliximab antibodies and relationship to clinical response in patients with rheumatoid arthritis. Arthritis Rheum 54(3):711–715. https://doi.org/10.1002/art.21671
Schellekens H (2002) Bioequivalence and the immunogenicity of biopharmaceuticals. Nat Rev Drug Discov 1(6):457–462. https://doi.org/10.1038/nrd818
Garcia Ruiz de Morales JM, Pascual-Salcedo D, Llinares Tello F, Valor Mendez L (2016) [Anti-tumor necrosis factor drug therapy: The usefulness of monitoring drug levels and anti-drug antibodies in clinical practice]. Med Clin (Barc) 147 (9):410–416. https://doi.org/10.1016/j.medcli.2016.04.002
Bandres Ciga S, Salvatierra J, Lopez-Sidro M, Garcia-Sanchez A et al (2015) An examination of the mechanisms involved in secondary clinical failure to adalimumab or etanercept in inflammatory arthropathies. J Clin Rheumatol 21(3):115–119. https://doi.org/10.1097/RHU.0000000000000229
Funding
This review was funded by a grant from Asociación para la Investigación en Reumatología de Marina Baixa (AIRE-MB).
Author information
Authors and Affiliations
Contributions
JCR conceived the project. MML and LC contributed to the design and implementation of the research, to the analysis of the results and to the writing of the manuscript. All authors discussed the results and were involved in the critical review of the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there is no competing interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Martín-López, M., Carmona, L., Balsa, A. et al. Serum drug levels of biologic agents in the management of rheumatoid arthritis and spondyloarthritis: a systematic review. Rheumatol Int 38, 975–983 (2018). https://doi.org/10.1007/s00296-018-4022-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-018-4022-4