Abstract
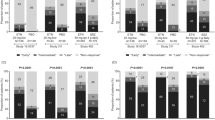
Despite the demonstrated efficacy of etanercept for the treatment of ankylosing spondylitis (AS), sulfasalazine is often prescribed, especially in countries with limited access to biologic agents. The objective of this subset analysis of the ASCEND trial was to compare the efficacy of etanercept and sulfasalazine in treating patients with AS from Asia, Eastern/Central Europe, and Latin America. A total of 287 patients, 190 receiving etanercept 50 mg once weekly and 97 receiving sulfasalazine 3 g daily, from eight countries were included in this subset analysis. Differences in disease activity and patient-reported outcomes assessing health-related quality-of-life (HRQoL) parameters in response to treatment were analyzed using the Cochran–Mantel–Haenszel test for categorical efficacy endpoints and analysis of covariance model for continuous variables. At week 16, a significantly greater proportion of patients receiving etanercept achieved ASAS20 (79.0 %) compared with patients receiving sulfasalazine (61.9 %; p = 0.002). At week 16, treatment with etanercept also resulted in significantly better responses than sulfasalazine for ASAS40 (64.7 vs. 35.1 %; p < 0.001), ASAS5/6 (48.1 vs. 26.3 %; p < 0.001), proportion of patients achieving 50 % response in Bath AS Disease Activity Index (65.8 vs. 42.3 %; p < 0.001), partial remission (35.3 vs. 17.5 %; p = 0.002), and all HRQoL parameters. Both treatments were well tolerated. Etanercept was significantly more effective than sulfasalazine in the treatment of patients with AS from Asia, Central/Eastern Europe, and Latin America.




Similar content being viewed by others
References
Sieper J, Braun J, Rudwaleit M, Boonen A, Zink A (2002) Ankylosing spondylitis: an overview. Ann Rheum Dis 61(Suppl 3):iii8–iii18
Braun J, Sieper J (2007) Ankylosing spondylitis. Lancet 369:1379–1390
Dean LE, Jones GT, MacDonald AG, Downham C, Sturrock RD, Macfarlane GJ (2014) Global prevalence of ankylosing spondylitis. Rheumatology 53:650–657
Peláez-Ballestas I, Navarro-Zarza JE, Julian B et al (2013) A community-based study on the prevalence of spondyloarthritis and inflammatory back pain in Mexicans. J Clin Rheumatol 19:57–61
Zochling J, van der Heijde D, Burgos-Vargas R et al (2006) ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 65:442–452
Braun J, Breban M, Maksymowych WP (2002) Therapy for ankylosing spondylitis: new treatment modalities. Best Pract Res Clin Rheumatol 16:631–651
Braun J, van der Horst-Bruinsma IE, Huang F et al (2011) Clinical efficacy and safety of etanercept versus sulfasalazine in patients with ankylosing spondylitis: a randomized, double-blind trial. Arthritis Rheum 63:1543–1551
Haibel H, Rudwaleit M, Brandt HC et al (2006) Adalimumab reduces spinal symptoms in active ankylosing spondylitis: clinical and magnetic resonance imaging results of a fifty-two-week open-label trial. Arthritis Rheum 54:678–681
van der Heijde D, Kivitz A, Schiff MH et al (2006) Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 54:2136–2146
Landewe R, Braun J, Deodhar A et al (2014) Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled phase 3 study. Ann Rheum Dis 73:39–47
Davis JC Jr, Van Der Heijde D, Braun J et al (2003) Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum 48:3230–3236
Calin A, Dijkmans BA, Emery P et al (2004) Outcomes of a multicentre randomised clinical trial of etanercept to treat ankylosing spondylitis. Ann Rheum Dis 63:1594–1600
Emery P, Fleischmann R, van der Heijde D et al (2011) The effects of golimumab on radiographic progression in rheumatoid arthritis: results of randomized controlled studies of golimumab before methotrexate therapy and golimumab after methotrexate therapy. Arthritis Rheum 63:1200–1210
Inman RD, Davis JC Jr, Heijde D et al (2008) Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase iii trial. Arthritis Rheum 58:3402–3412
Braun J, Brandt J, Listing J et al (2002) Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet 359:1187–1193
van der Heijde D, Dijkmans B, Geusens P et al (2005) Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (assert). Arthritis Rheum 52:582–591
Braun J, van den Berg R, Baraliakos X et al (2011) 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 70:896–904
Anderson JJ, Baron G, van der Heijde D, Felson DT, Dougados M (2001) Ankylosing spondylitis assessment group preliminary definition of short-term improvement in ankylosing spondylitis. Arthritis Rheum 44:1876–1886
Brandt J, Listing J, Sieper J, Rudwaleit M, van der Heijde D, Braun J (2004) Development and preselection of criteria for short term improvement after anti-TNF alpha treatment in ankylosing spondylitis. Ann Rheum Dis 63:1438–1444
Jenkinson TR, Mallorie PA, Whitelock HC, Kennedy LG, Garrett SL, Calin A (1994) Defining spinal mobility in ankylosing spondylitis (as). The bath as metrology index. J Rheumatol 21:1694–1698
Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A (1994) A new approach to defining disease status in ankylosing spondylitis: the bath ankylosing spondylitis disease activity index. J Rheumatol 21:2286–2291
Calin A, Garrett S, Whitelock H et al (1994) A new approach to defining functional ability in ankylosing spondylitis: the development of the bath ankylosing spondylitis functional index. J Rheumatol 21:2281–2285
Godfrin-Valnet M, Prati C, Puyraveau M, Toussirot E, Letho-Gyselink H, Wendling D (2013) Evaluation of spondylarthritis activity by patients and physicians: asdas, basdai, pass, and flares in 200 patients. Joint Bone Spine 80:393–398
The EuroQol Group (1990) Euroqol—a new facility for the measurement of health-related quality of life. Health Policy 16:199–208
Ware JE, Kosinski M (2001) Interpreting SF-36 summary health measures: a response. Qual Life Res 10:405–413; discussion 415–420
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67:361–370
Péntek M, Poór G, Wiland P et al (2014) Biological therapy in inflammatory rheumatic diseases: issues in central and eastern european countries. Eur J Health Econ 15(Suppl 1):S35–S43
Rachid B, El Zorkany B, Youseif E, Tikly M (2012) Early diagnosis and treatment of ankylosing spondylitis in Africa and the middle east. Clin Rheumatol 31:1633–1639
Hammoudeh M, Alarfaj A, Chen DY, Djoudi H, Youseif E, Zhu J (2013) Safety of tumor necrosis factor inhibitors use for rheumatoid arthritis and ankylosing spondylitis in Africa, the middle east, and Asia: focus on severe infections and tuberculosis. Clin Rheumatol 32:293–300
Benhamou M, Gossec L, Dougados M (2010) Clinical relevance of c-reactive protein in ankylosing spondylitis and evaluation of the NSAIDs/coxibs’ treatment effect on c-reactive protein. Rheumatology 49:536–541
de Vlam K (2010) Soluble and tissue biomarkers in ankylosing spondylitis. Best Pract Res Clin Rheumatol 24:671–682
Sanders KM, Hertzman A, Escobar MR, Littman BH (1987) Correlation of immunoglobulin and c reactive protein levels in ankylosing spondylitis and rheumatoid arthritis. Ann Rheum Dis 46:273–276
Vastesaeger N, van der Heijde D, Inman RD et al (2011) Predicting the outcome of ankylosing spondylitis therapy. Ann Rheum Dis 70:973–981
Khan NA, Spencer HJ, Abda E et al (2012) Determinants of discordance in patients’ and physicians’ rating of rheumatoid arthritis disease activity. Arthritis Care Res (Hoboken) 64:206–214
Markenson JA, Koenig AS, Feng JY et al (2013) Comparison of physician and patient global assessments over time in patients with rheumatoid arthritis: a retrospective analysis from the radius cohort. J Clin Rheumatol 19:317–323
Acknowledgments
Medical writing support was provided by Mukund Nori, PhD, MBA, CMPP, of Engage Scientific Solutions and was funded by Pfizer, New York, NY, USA.
Funding
This research was funded by Pfizer, New York, NY, USA.
Author contributions
ND, WAS, FH, KS, LJQL, EB, RB-V, SK, and EM have made substantial contributions to the conception and design of this subset analysis. AS provided all the statistical analyses for this manuscript. All authors contributed substantially to the interpretation of data presented, drafting the manuscript, and revising it critically for important intellectual content and have given final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that issues related to either accuracy or integrity of any part of the manuscript are appropriately addressed and resolved.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author ND declares that there is no conflict of interest. Author WAS declares that there is no conflict of interest. Author FH declares that there is no conflict of interest. Author SK is an employee of Pfizer. Author RB-V has been on the speakers’ bureau and advisory boards of Abbvie, Janssen, Pfizer, and UCB. Author KS is an employee of Pfizer. Author EB is an employee of Pfizer. Author AS is an employee of Inventive Health, who was a paid contractor to Pfizer in the development of this manuscript. Author LJQL is an employee of Pfizer. Author EB is an employee of Pfizer. All authors declare that they have no other competing interests (neither financial competing interests nor political, personal, religious, ideological, academic, intellectual, commercial, or any other) in relation to this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Damjanov, N., Shehhi, W.A., Huang, F. et al. Assessment of clinical efficacy and safety in a randomized double-blind study of etanercept and sulfasalazine in patients with ankylosing spondylitis from Eastern/Central Europe, Latin America, and Asia. Rheumatol Int 36, 643–651 (2016). https://doi.org/10.1007/s00296-016-3452-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-016-3452-0