Abstract
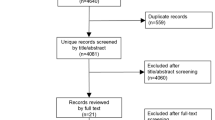
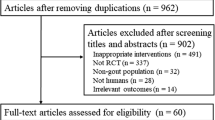
Allopurinol is the most widely used urate-lowering drug (ULD). Together with efficacy and cost, safety is an aspect that helps taking clinical decisions. This systematic review analyzes allopurinol safety. The literature search was performed in MEDLINE, EMBASE, and the Cochrane Library (January 2014). Selection criteria: (a) patients >18, (b) gout by the ACR criteria or evidence of urate crystal in synovial fluid, (c) comparator (placebo or other ULD), and (d) RCTs, cohorts, or meta-analysis. Primary outcomes: rate of adverse events and death. The quality was assessed with the Jadad’s scale. A meta-analysis with fixed effects was performed. From 544 studies, seven met the eligibility criteria and were included. All RCT presented a low power for safety. All RCTs included a mixed population of patients with gout and hyperuricemia. Allopurinol (300 mg) was compared to febuxostat (40–240 mg) in five RCTs, to benzbromarone and probenecid in two RCTs, and to placebo in one. In the RCTs comparing allopurinol with benzbromarone and probenecid, the highest discontinuation rate was with probenecid (26 %), followed by allopurinol (11 %) and benzbromarone (4 %). The incidence of adverse events was similar between allopurinol (range 38.6–85) and febuxostat (range 41.8–80). Six patients on febuxostat and three on allopurinol died during the studies; no deaths were judged related to drug. The combined risk of adverse events was RR = 1.04 (95 % CI 0.98, 1.11). Allopurinol is a safe option, slightly better than other ULDs. The grade of evidence is high, but further research is needed to evaluate higher doses and long-term safety.


Similar content being viewed by others
References
Tausche AK, Jansen TL, Schroder HE, Bornstein SR, Aringer M, Muller-Ladner U (2009) Gout—current diagnosis and treatment. Deutsches Arzteblatt Int 106(34–35):549–555. doi:10.3238/arztebl.2009.0549
Weaver AL (2008) Epidemiology of gout. Cleve Clin J Med 75(Suppl 5):S9–S12
Lipworth W, Kerridge I, Brett J, Day R (2011) How clinical and research failures lead to suboptimal prescribing: the example of chronic gout. BMJ 343:d7459. doi:10.1136/bmj.d7459
Schlesinger N (2004) Management of acute and chronic gouty arthritis: present state-of-the-art. Drugs 64(21):2399–2416
Perez-Ruiz F, Calabozo M, Pijoan JI, Herrero-Beites AM, Ruibal A (2002) Effect of urate-lowering therapy on the velocity of size reduction of tophi in chronic gout. Arthritis Rheum 47(4):356–360. doi:10.1002/art.10511
Stamp LK, Taylor WJ, Jones PB, Dockerty JL, Drake J, Frampton C, Dalbeth N (2012) Starting dose is a risk factor for allopurinol hypersensitivity syndrome: a proposed safe starting dose of allopurinol. Arthritis Rheum 64(8):2529–2536. doi:10.1002/art.34488
Hande KR, Noone RM, Stone WJ (1984) Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med 76(1):47–56
Stamp LK, O’Donnell JL, Zhang M, James J, Frampton C, Barclay ML, Chapman PT (2011) Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum 63(2):412–421. doi:10.1002/art.30119
Edwards NL (2009) Febuxostat: a new treatment for hyperuricaemia in gout. Rheumatology (Oxford) 48(Suppl 2):ii15–ii19. doi:10.1093/rheumatology/kep088
Pascual E, Sivera F (2007) Therapeutic advances in gout. Curr Opin Rheumatol 19(2):122–127. doi:10.1097/BOR.0b013e32802106b9
van Tulder M, Furlan A, Bombardier C, Bouter L, Editorial Board of the Cochrane Collaboration Back Review G (2003) Updated method guidelines for systematic reviews in the Cochrane collaboration back review group. Spine 28(12):1290–1299. doi:10.1097/01.BRS.0000065484.95996.AF
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17(1):1–12
Stevenson M, Pandor A (2011) Febuxostat for the management of hyperuricaemia in patients with gout: a NICE single technology appraisal. Pharmacoeconomics 29(2):133–140. doi:10.2165/11535770-000000000-00000
Stevenson M, Pandor A (2009) Febuxostat for the treatment of hyperuricaemia in people with gout: a single technology appraisal. Health Technol Assess 13(Suppl 3):37–42. doi:10.3310/hta13suppl3/06
Yu KH (2007) Febuxostat: a novel non-purine selective inhibitor of xanthine oxidase for the treatment of hyperuricemia in gout. Recent Pat Inflamm Allergy Drug Discov 1(1):69–75
Singh JA (2010) Advances in gout: some answers, more questions. Arthritis Res Ther 12(5):136. doi:10.1186/ar3110
Schumacher HR Jr, Becker MA, Wortmann RL, Macdonald PA, Hunt B, Streit J, Lademacher C, Joseph-Ridge N (2008) Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum 59(11):1540–1548. doi:10.1002/art.24209
Reinders MK, Haagsma C, Jansen TL, van Roon EN, Delsing J, van de Laar MA, Brouwers JR (2009) A randomised controlled trial on the efficacy and tolerability with dose escalation of allopurinol 300–600 mg/day versus benzbromarone 100–200 mg/day in patients with gout. Ann Rheum Dis 68(6):892–897. doi:10.1136/ard.2008.091462
Reinders MK, van Roon EN, Jansen TL, Delsing J, Griep EN, Hoekstra M, van de Laar MA, Brouwers JR (2009) Efficacy and tolerability of urate-lowering drugs in gout: a randomised controlled trial of benzbromarone versus probenecid after failure of allopurinol. Ann Rheum Dis 68(1):51–56. doi:10.1136/ard.2007.083071
Becker MA, Schumacher HR, Espinoza LR, Wells AF, MacDonald P, Lloyd E, Lademacher C (2010) The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther 12(2):R63. doi:10.1186/ar2978
Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, Streit J, Joseph-Ridge N (2005) Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 353(23):2450–2461. doi: doi:10.1056/NEJMoa050373
Kamatani N, Fujimori S, Hada T, Hosoya T, Kohri K, Nakamura T, Ueda T, Yamamoto T, Yamanaka H, Matsuzawa Y (2011) Multicenter, open-label study of long-term administration of febuxostat (TMX-67) in Japanese patients with hyperuricemia including gout. J Clin Rheumatol 17(4 Suppl 2):S50–S56. doi:10.1097/RHU.0b013e31822541d0
Kamatani N, Fujimori S, Hada T, Hosoya T, Kohri K, Nakamura T, Ueda T, Yamamoto T, Yamanaka H, Matsuzawa Y (2011) An allopurinol-controlled, randomized, double-dummy, double-blind, parallel between-group, comparative study of febuxostat (TMX-67), a non-purine-selective inhibitor of xanthine oxidase, in patients with hyperuricemia including those with gout in Japan: phase 3 clinical study. J Clin Rheumatol 17(4 Suppl 2):S13–S18. doi:10.1097/RHU.0b013e31821d36cc
Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu TF (1977) Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum 20(3):895–900
Wyngaarden JB, Rundles RW, Metz EN (1965) Allopurinol in the treatment of gout. Ann Intern Med 62:842–847
Acknowledgments
This work was supported by the Spanish Society of Rheumatology and Menarini. Menarini had no role in the study design, literature search, data collection, data analysis, data interpretation, or writing of this report.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: MEDLINE search
P (patients) | Search ((“Gout”[Mesh]) OR Gouts OR “Arthritis, Gouty”[Mesh] OR Gouty Arthritis OR Arthritides, Gouty OR Gouty Arthritides) OR (“Juvenile gout”[Supplementary Concept] OR Gouty nephropathy, familial juvenile OR Nephropathy, familial, with gout OR Familial juvenile hyperuricaemic nephropathy) OR (acute gout) OR (gouty) OR (gouty arthritis) OR (acute gouty arthritis) OR (tophaceous gout) OR (chronic tophaceous gout) OR (chronic tophaceous gout) OR (gout hyperuricemia) OR (gout renal) OR (hyperuricemia gout) OR (gout arthritis) OR (chronic gout) OR (“Hyperuricemia”[Mesh]) OR (“Uric Acid”[Mesh] OR Acid, Uric OR Trioxopurine OR 2,6,8-Trihydroxypurine OR Potassium Urate OR Urate, Potassium OR Urate OR Ammonium Acid Urate OR Acid Urate, Ammonium OR Urate, Ammonium Acid OR Sodium Urate Monohydrate OR Monohydrate, Sodium Urate OR Urate Monohydrate, Sodium OR Monosodium Urate Monohydrate OR Monohydrate, Monosodium Urate OR Urate Monohydrate, Monosodium OR Sodium Acid Urate Monohydrate OR Sodium Urate OR Urate, Sodium OR Monosodium Urate OR Urate, Monosodium OR Sodium Acid Urate OR Acid Urate, Sodium OR Urate, Sodium Acid) OR (intercritical gout) OR (monohydrate crystals) OR (primary gout) OR (secondary gout) |
I (intervention) | Search “Allopurinol”[Mesh] OR Zyloprim OR Wellcome Brand of Allopurinol OR Allopurinol Wellcome Brand OR Zyloric OR Glaxo Wellcome Brand of Allopurinol OR Allopurinol OR Bicther Brand of Allopurinol OR Allopurinol Bicther Brand OR Allorin OR Douglas Brand of Allopurinol OR Allopurinol Douglas Brand OR Allpargin OR Merz Brand of Allopurinol OR Allopurinol Merz Brand OR Allural OR Pan Quimica OR Quimica, Pan OR Apulonga OR Dorsch Brand of Allopurinol OR Allopurinol Dorsch Brand OR Apurin OR Multipharma Brand of Allopurinol OR Allopurinol Multipharma Brand OR Atisuril OR Byk Gulden Brand of Allopurinol OR Bleminol OR gepepharm Brand of Allopurinol OR Allopurinol gepepharm Brand OR Caplenal OR Rhône-Poulenc Rorer Brand of Allopurinol OR Rhône-Poulenc Rorer Brand of Allopurinol OR APS Brand of Allopurinol OR Allopurinol APS Brand OR Capurate OR Fawns |
0 (outcome) | Search ((((((((((((((((((((((“adverse effects “[Subheading] OR side effects OR undesirable effects OR injurious effects)) OR (“Safety”[Mesh] OR Safeties)) OR (“Drug Toxicity”[Mesh] OR Drug Toxicities OR Toxicities, Drug OR Toxicity, Drug OR Drug Safety OR Safety, Drug OR Adverse Drug Reaction OR Adverse Drug Reactions OR Drug Reaction, Adverse OR Drug Reactions, Adverse OR Reaction, Adverse Drug OR Reactions, Adverse Drug OR Adverse Drug Event OR Adverse Drug Events OR Drug Event, Adverse OR Drug Events, Adverse OR Event, Adverse Drug OR Events, Adverse Drug)) OR (“toxicity “[Subheading] OR toxic potential OR margin of safety)) OR (drug fatality)) OR (‘drug mortality’ OR ‘fatal adverse drug reaction’ OR ‘fatal adverse reaction’ OR ‘fatal side effect’)) OR (drug mortality OR fatal adverse drug reaction OR fatal adverse reaction OR fatal side effect)) OR (“poisoning “[Subheading] OR poisonous effects)) OR (“Drug Hypersensitivity”[Mesh] OR Drug Hypersensitivities OR Hypersensitivities, Drug OR Drug Allergy OR Allergies, Drug OR Drug Allergies OR Hypersensitivity, Drug OR Allergy, Drug)) OR (‘drug sensitivity’ OR ‘drug sensitivity test’ OR ‘drug subsensitivity’ OR ‘drug susceptibility’ OR ‘parasitic sensitivity tests’ OR ‘susceptibility, drug’)) OR (drug sensitivity OR drug sensitivity test OR drug subsensitivity OR drug susceptibility OR parasitic sensitivity tests OR susceptibility, drug)) OR (sensitivity drug)) OR (“Drug Interactions”[Mesh] OR Drug Interaction OR Interaction, Drug OR Interactions, Drug)) OR (“drug effects “[Subheading] OR pharmacologic effects OR effect of drugs)) OR (“Adverse Drug Reaction Reporting Systems”[Mesh] OR Drug Reaction Reporting Systems, Adverse)) OR (‘adverse drug reaction’ OR ‘adverse drug effect’ OR ‘adverse drug eventor adverse effect’ OR ‘adverse reaction’ OR ‘adverse reaction, drug’ OR ‘drug adverse effect’ OR ‘drug adverse reaction’ OR ‘drug reaction, adverse’ OR ‘drug side effect’)) OR (‘adverse drug reaction’ OR ‘adverse drug effect’ OR “adverse drug eventor” OR “adverse effect” OR ‘adverse reaction’ OR ‘adverse reaction, drug’ OR ‘drug adverse effect’ OR ‘drug adverse reaction’ OR ‘drug reaction, adverse’ OR ‘drug side effect’)) OR (adverse drug reaction OR adverse drug effect OR “adverse drug eventor” OR “adverse effect” OR adverse reaction OR adverse reaction, drug OR drug adverse effect OR drug adverse reaction OR drug reaction, adverse OR drug side effect)) OR (“drug carcinogenicity” OR ‘carcinogenicity, drug induced’)) OR (“drug carcinogenicity” OR carcinogenicity, drug induced)) OR (“drug cytotoxicity” OR “cytotoxicity, drug”)) OR (“Treatment Outcome”[Mesh] OR Outcome, Treatment OR Rehabilitation Outcome OR Outcome, Rehabilitation OR Treatment Effectiveness OR Effectiveness, Treatment OR Treatment Efficacy OR Efficacy, Treatment) OR “Hypersensitivity”[Mesh] OR Hypersensitivities OR Allergy OR Allergies OR Allergic Reaction OR Allergic Reactions OR Reaction, Allergic OR Reactions, Allergic OR Search “Stevens-Johnson Syndrome”[Mesh] OR Stevens Johnson Syndrome OR Search (((“Allopurinol/adverse effects”[Mesh]) OR ( “Gout Suppressants/adverse effects”[Mesh] OR “Gout Suppressants/toxicity”[Mesh])) OR ( “Drug Eruptions/chemically induced”[Mesh] OR “Drug Eruptions/complications”[Mesh] ))OR(“Eosinophilia/chemically induced”[Mesh]OR”Eosinophilia/complications”[Mesh]) OR DRESS OR “drug related eosinophilia with systemic symptoms” |
Study | Search ((((((((((((((“Review”[Publication Type] OR Review, Systematic OR Review, Multicase OR Review Literature OR Review, Academic OR Review of Reported Cases OR Review)) OR (((“Clinical Trial”[Publication Type]) OR “Validation Studies”[Publication Type]) OR “Evaluation Studies”[Publication Type])) OR (“Clinical Trial, Phase I”[Publication Type] OR Clinical Trial, Phase 1)) OR (“Clinical Trial, Phase II”[Publication Type] OR Clinical Trial, Phase 2 OR Clinical Trial, Phase II)) OR (“Clinical Trial, Phase III”[Publication Type] OR Clinical Trial, Phase 3 OR Clinical Trial, Phase III)) OR (“Clinical Trial, Phase IV”[Publication Type] OR Clinical Trial, Phase 4 OR Clinical Trial, Phase IV)) OR (“Controlled Clinical Trial”[Publication Type])) OR (“Multicenter Study”[Publication Type] OR Multicenter Studies OR Multicenter Study)) OR (“Randomized Controlled Trial”[Publication Type] OR Randomized Controlled Trial)) OR (“Cohort Studies”[Mesh] OR Cohort Study OR Studies, Cohort OR Study, Cohort OR Concurrent Studies OR Studies, Concurrent OR Concurrent Study OR Study, Concurrent OR Historical Cohort Studies OR Studies, Historical Cohort OR Cohort Studies, Historical OR Cohort Study, Historical OR Historical Cohort Study OR Study, Historical Cohort OR Analysis, Cohort OR Analyses, Cohort OR Cohort Analyses OR Cohort Analysis OR Closed Cohort Studies OR Cohort Studies, Closed OR Closed Cohort Study OR Cohort Study, Closed OR Study, Closed Cohort OR Studies, Closed Cohort OR Incidence Studies OR Incidence Study OR Studies, Incidence OR Study, Incidence OR Cohort Studies)) OR (“Cohort Studies”[Mesh] OR cohort study OR studies, cohort OR study, cohort OR concurrent studies OR studies, concurrent OR concurrent study OR study, concurrent OR historical cohort studies OR studies, historical cohort OR cohort studies, historical OR cohort study, historical OR historical cohort study OR study, historical cohort OR analysis, cohort OR analysis, cohort OR cohort analyses OR cohort analysis OR closed cohort studies OR cohort studies, closed OR closed cohort study OR cohort study, closed OR study, closed cohort OR studies, closed cohort OR incidence studies OR incidence study OR studies, incidence OR study, incidence OR cohort studies)) OR (“Longitudinal Studies”[Mesh] OR Longitudinal Study OR Studies, Longitudinal OR Study, Longitudinal OR Longitudinal Survey OR Longitudinal Surveys OR Survey, Longitudinal OR Surveys, Longitudinal OR Longitudinal Studies)) OR (“Follow-Up Studies”[Mesh] OR Follow-Up Studies OR Follow-Up Study OR Studies, Follow-Up OR Study, Follow-Up OR Follow-up Studies OR Follow-up Study OR Studies, Follow-up OR Study, Follow-up OR Follow-Up Studies)) |
Limits | Humans, English, French, Spanish |
Appendix 2: Excluded studies and reason for exclusion
Article | Reason for exclusion |
|---|---|
Kim (2013) | Incidence of cutaneous adverse reactions in allopurinol users |
White (2012) | Gout patients at high risk of CV events |
Wells (2012) | Secondary analysis of the CONFIRMS trial comparing Afro-Americans with Caucasian |
Tayar (2012) | Febuxostat review and meta-analysis |
Chohan (2012) | Comparison of female versus male gout patients |
Schumacher (2012) | Rilonacept and allopurinol versus placebo |
Becker (2011) | Side effects depending on patient age not treatment |
Suarez-Almazor (2010) | Protocol to review febuxostat use in gout |
Saito (2010), Hanvivadhanakul (2002), Stolbach (1982) | No gout patients |
Poon (2009), Luk (2009), Sundy (2007), Perez-Ruiz (1998), Gibson (1982) | No data about allopurinol safety |
Reinders (2007) | Allopurinol versus allopurinol and probenecid |
Keenan (2012), Kim (2006), Wortmann (2005), Pascual (2000), Pascual (2007) | Narrative reviews |
Catton (2006) | Protocol to review allopurinol use in gout |
Pohar (2006) | Canadian recommendation to use febuxostat |
Takahashi (2003) | Allopurinol was not included as treatment arm |
Vazquez-Mellado (2001) | Prevalence study of side effect of allopurinol in patients with gout |
Perez-Ruiz (1999) | Patients with gout and renal function impairment |
Delbarre (1966), Kuzell (1966) | Case reports |
Appendix 3: Characteristics of the included reviews and meta-analysis
Review and meta-analysis | |
|---|---|
Stevenson (2011) Evidence Review Group(ERG) NICE recommendations | Includes: Becker (2005) Schumacher ACR-1837 (2005) |
Singh (2010) Meta-analysis of allopurinol and febuxostat safety | Includes: Becker (2010) Schumacher (2008) Becker (2005) |
Stevenson (2009) Evidence Review Group(ERG) NICE recommendations | Includes: Becker (2005) Schumacher ACR-1837 (2005) |
Yu (2007) Review about Febuxostat efficacy | Includes: Becker (2005) Schumacher ACR-1837 (2005) |
Rights and permissions
About this article
Cite this article
Castrejon, I., Toledano, E., Rosario, M.P. et al. Safety of allopurinol compared with other urate-lowering drugs in patients with gout: a systematic review and meta-analysis. Rheumatol Int 35, 1127–1137 (2015). https://doi.org/10.1007/s00296-014-3189-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-014-3189-6