Abstract
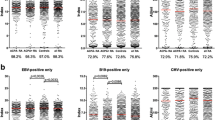
Antibody to Epstein–Barr virus (EBV) early antigen diffuse (anti-EA-D) is associated with viral replication. However, their possible associations with clinical/therapeutic features in primary Sjögren’s syndrome (pSS) were not established. We evaluated 100 pSS patients (American–European Criteria) and 89 age/gender/ethnicity-matched healthy controls. Disease activity was measured by EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI). Antibodies to EBV (anti-VCA IgG/IgM, anti-EBNA-1 IgG, anti-EA-D IgG) were determined by ELISA. Patients and controls had comparable frequencies and mean levels of anti-VCA IgG (90 vs. 86.5 %, p = 0.501; 2.6 ± 1.1 vs. 2.5 ± 1.1 AU/mL, p = 0.737) and anti-EBNA-1 IgG (92 vs. 94.4 %, p = 0.576; 141.3 ± 69.8 vs. 135.6 ± 67.5 RU/mL, p = 0.464). Anti-VCA IgM was negative in all cases. Noteworthy, higher frequency and increased mean levels of anti-EA-D were observed in patients than controls (36 vs. 4.5 %, p < 0.0001; 38.6 ± 57.4 vs. 7.9 ± 26.3 RU/mL, p < 0.0001). Further analysis of patients with (n = 36) and without (n = 64) anti-EA-D revealed comparable age/gender/ethnicity (p ≥ 0.551), current prednisone dose (4.8 ± 6.9 vs. 5.1 ± 10.4 mg/day, p = 0.319), and current uses of prednisone (52.8 vs. 37.5 %, p = 0.148) and immunosuppressants (44.4 vs. 31.3 %, p = 0.201). ESSDAI values were comparable (p = 0.102), but joint activity was more frequent (25 vs. 9.4 %, p = 0.045) in anti-EA-D positive patients. Anti-EA-D antibodies were not associated with anti-Ro/SSA (p = 1.000), anti-La/SSB (p = 0.652), rheumatoid factor (p = 1.000), anti-α-fodrin (p = 0.390) or antiphospholipid antibodies (p = 0.573), not suggesting cross-reactivity. The higher anti-EA-D frequency associated with joint activity raises the possibility that a subclinical EBV reactivation may trigger or perpetuate the articular involvement in pSS.

Similar content being viewed by others
References
Tzioufas AG, Voulgarelis M (2007) Update on Sjögren’s syndrome autoimmune epithelitis: from classification to increased neoplasias. Best Pract Res Clin Rheumatol 21:989–1010
Amarasena R, Bowman S (2007) Sjögren’s syndrome. Clin Med 7:53–56
García-Carrasco M, Ramos-Casals M, Rosas J et al (2002) Primary Sjögren syndrome: clinical and immunologic disease patterns in a cohort of 400 patients. Medicine 81:270–280
Nardi N, Brito-Zerón P, Ramos-Casals M et al (2006) Circulating auto-antibodies against nuclear and non-nuclear antigens in primary Sjögren’s syndrome. Prevalence and clinical significance in 335 patients. Clin Rheumatol 25:341–346
Routsias JG, Tzioufas AG (2007) Sjögren’s syndrome- study of autoantigens and autoantibodies. Clinic Rev Allerg Immunol 32:238–251
Vitali C, Bombardieri S, Jonsson R et al (2002) Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 61:554–558
James JA, Harley JB, Scofield RH (2001) Role of viruses in systemic lupus erythematosus and Sjögren syndrome. Curr Opin Rheumatol 13:370–376
Toussirot E, Roudier J (2008) Epstein–Barr virus in autoimmune diseases. Best Pract Res Clin Rheumatol 22:883–896
Poole BD, Scofield RH, Harley JB, James JA (2006) Epstein–Barr virus and molecular mimicry in systemic lupus erythematosus. Autoimmunity 39:63–70
Origgi L, Hu C, Bertetti E et al (1988) Antibodies to Epstein–Barr virus and cytomegalovirus in primary Sjogren’s syndrome. Boll Ist Sieroter Milan 67:265–274
Yamaoka K, Miyasaka N, Yamamoto K (1988) Possible involvement of Epstein–Barr virus in polyclonal B cell activation in Sjögren’s syndrome. Arthritis Rheum 31:1014–1021
Miyasaka N, Yamaoka K, Tateishi M, Nishioka K, Yamamoto K (1989) Possible involvement of Epstein–Barr virus (EBV) in polyclonal B-cell activation in Sjögren’s syndrome. J Autoimmun 2:427–432
Mariette X, Gozlan J, Clerc D, Bisson M, Morinet F (1991) Detection of Epstein–Barr virus DNA by in situ hybridization and polymerase chain reaction in salivary gland biopsy specimens from patients with Sjögren’s syndrome. Am J Med 90:286–294
Perrot S, Calvez V, Escande JP, Dupin N, Marcelin AG (2003) Prevalences of herpesviruses DNA sequences in salivary gland biopsies from primary and secondary Sjögren’s syndrome using degenerated consensus PCR primers. J Clin Virol 28:165–168
Pflugfelder SC, Crouse C, Pereira I, Atherton S (1990) Amplification of Epstein–Barr virus genomic sequences in blood cells, lacrimal glands, and tears from primary Sjögren’s syndrome patients. Ophthalmology 97:976–984
Newkirk MM, Shiroky JB, Johnson N et al (1996) Rheumatic disease patients, prone to Sjögren’s syndrome and/or lymphoma, mount an antibody response to BHRF1, the Epstein-Barr viral homologue of BCL-2. Br J Rheumatol 35:1075–1081
Yang EV, Webster Marketon JI, Chen M, Lo KW, Kim SJ, Glaser R (2010) Glucocorticoids activate Epstein Barr virus lytic replication through the upregulation of immediate early BZLF1 gene expression. Brain Behav Immun 24:1089–1096
Feng WH, Cohen JI, Fischer S et al (2004) Reactivation of latent Epstein–Barr virus by methotrexate: a potential contributor to methotrexate-associated lymphomas. J Natl Cancer Inst 96:1691–1702
Seror R, Ravaud P, Bowman SJ et al (2010) EULAR Sjogren’s syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjogren’s syndrome. Ann Rheum Dis 69:1103–1109
Mitchell JL, Doyle CM, Land MV, Devine PL (1998) Comparison of commercial ELISA for detection of antibodies to the viral capsid antigen (VCA) of Epstein–Barr virus (EBV). Dis Markers 13:245–249
De Paschale M, Cagnin D, Cerulli T et al (2010) Search for anti-EA(D) antibodies in subjects with an “isolated VCA IgG” pattern. Int J Microbiol 2010:1–4
Berini CA, Susana Pascuccio M, Bautista CT et al (2008) Comparison of four commercial screening assays for the diagnosis of human T-cell lymphotropic virus types 1 and 2. J Virol Methods 147:322–327
Esparza RH, Swaak T, Aarden L, Smeenk R (1985) Complement-fixing antibodies to dsDNA detected by the immunofluorescence technique on Crithidia luciliae. A critical appraisal. J Rheumatol 12:1109–1117
Mahler M, Stinton LM, Fritzler MJ (2005) Improved serological differentiation between systemic lupus erythematosus and mixed connective tissue disease by use of a SmD3 peptide-based immunoassay. Clin Diagn Lab Immunol 12:107–113
Ruiz-Tíscar JL, López-Longo FJ, Sánchez-Ramón S et al (2005) Prevalence of IgG anti-{alpha}-fodrin antibodies in Sjogren’s syndrome. Ann N Y Acad Sci 1050:210–216
Goeldner I, Skare TL, de Messias Reason IT, Nisihara RM, Silva MB, Utiyama SR (2010) Anti-cyclic citrullinated peptide antibodies and rheumatoid factor in rheumatoid arthritis patients and relatives from Brazil. Rheumatology (Oxford) 49:1590–1593
Bakker AJ, Slomp J, de Vries T et al (2003) Adequate sampling in cryoglobulinaemia: recommended warmly. Clin Chem Lab Med 41:85–89
Harris EN, Pierangeli S (1991) The anticardiolipin ELISA test. Clin Immunol Newsletter 11:33–44
Miyakis S, Lockshin MD, Atsumi T et al (2006) International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 4:295–306
Haeri S, Baker AM, Boggess KA (2010) Prevalence of Epstein–Barr virus reactivation in pregnancy. Am J Perinatol 27:715–719
Macsween KF, Crawford DH (2003) Epstein–Barr virus-recent advances. Lancet Infect Dis 3:131–140
Quinlivan EB, Holley-Guthrie EA, Norris M, Gutsch D, Bachenheimer SL, Kenney SC (1993) Direct BRLF1 binding is required for cooperative BZLF1/BRLF1 activation of the Epstein–Barr virus early promoter, BMRF1. Nucleic Acids Res 21:1999–2007
National Center for Infectious Diseases, Centers for Disease Control and Prevention (2006) Epstein–Barr virus and infectious mononucleosis. www.cdc.gov/ncidod/diseases/ebv.htm. Updated: 05/16/2006. The web page has been accessed in October/2011
Glaser R, Strain EC, Tarr KL, Holliday JE, Donnerberg RL, Kiecolt-Glaser JK (1985) Changes in Epstein–Barr virus antibody titers associated with aging. Proc Soc Exp Biol Med 179:352–355
Wagner HJ, Hornef M, Teichert HM, Kirchner H (1994) Sex difference in the serostatus of adults to the Epstein–Barr virus. Immunobiology 190:424–429
Parks CG, Cooper GS, Hudson LL et al (2005) Association of Epstein–Barr virus with systemic lupus erythematosus: effect modification by race, age, and cytotoxic T lymphocyte-associated antigen 4 genotype. Arthritis Rheum 52:1148–1159
de-Thé G (1976) Epstein–Barr virus behavior in different populations and implications for control of Epstein–Barr virus-associated tumors. Cancer Res 36:692–695
McClain MT, Heinlen LD, Dennis GJ, Roebuck J, Harley JB, James JA (2005) Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nat Med 11:85–89
Zandman-Goddard G, Berkun Y, Barzilai O et al (2009) Exposure to Epstein–Barr virus infection is associated with mild systemic lupus erythematosus disease. Ann N Y Acad Sci 1173:658–663
Esen BA, Yilmaz G, Uzun S et al (2012) Serologic response to Epstein–Barr virus antigen in patients with systemic lupus erythematosus: a controlled study. Rheumatol Int 32:79–83
Harley JB, Harley IT, Guthridge JM, James JA (2006) The curiously suspicious: a role for Epstein–Barr virus in lupus. Lupus 15:768–777
Kuwana Y, Takei M, Yajima M et al (2011) Epstein–Barr virus induces erosive arthritis in humanized mice. PLoS One 6: e26630 (Epub 2011 Oct 19)
Terada K, Katamine S, Eguchi K et al (1994) Prevalence of serum and salivary antibodies to HTLV-1 in Sjögren’s syndrome. Lancet 344:1116–1119
Mariette X, Agbalika F, Zucker-Franklin D et al (2000) Detection of the tax gene of HTLV-I in labial salivary glands from patients with Sjögren’s syndrome and other diseases of the oral cavity. Clin Exp Rheumatol 18:341–347
Segurado AA, Malaque CM, Sumita LM, Pannuti CS, Lal RB (1997) Laboratory characterization of human T cell lymphotropic virus types 1 (HTLV-1) and 2 (HTLV-2) infections in blood donors from Sao Paulo, Brazil. Am J Trop Med Hyg 57:142–148
Morofuji-Hirata M, Kajiyama W, Nakashima K, Noguchi A, Hayashi J, Kashiwagi S (1993) Prevalence of antibody to human T-cell lymphotropic virus type I in Okinawa, Japan, after an interval of 9 years. Am J Epidemiol 137:43–48
Acknowledgments
This work was supported by Agency for Promotion of Research, Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) # 2010/10013-4, 2010/10017-0 to RRN, 2010/13463-0 to HPC, and Conselho Nacional de Pesquisa (CNPQ) # 301411/2009-3 to EB. We thank the biomedicals Angela Maria Egydio Barreto e Maria Aparecida de Oliveira Bellesa of the Blood Bank (Fundação Pró-Sangue-Hemocentro) for their help in confirmation of positive sera for HTLV and the staff of the Laboratory of the Hospital das Clínicas da Faculdade de Medicina da USP for carrying out the research of anti-VCA, HTLV serology (ELISA), lupus anticoagulant and determination of serum levels of IgG.
Conflict of interest
None of the authors has any conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pasoto, S.G., Natalino, R.R., Chakkour, H.P. et al. EBV reactivation serological profile in primary Sjögren’s syndrome: an underlying trigger of active articular involvement?. Rheumatol Int 33, 1149–1157 (2013). https://doi.org/10.1007/s00296-012-2504-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-012-2504-3