Abstract
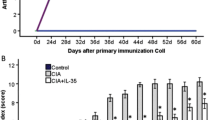
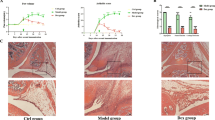
This study was performed to investigate the effects of IL-32 on joint inflammation, bone destruction, and synovial cytokine expressions, and on synovial natural killer (NK) cell expressions in collagen-induced arthritis (CIA). CIA was induced by type II collagen in DBA1 mice, and phosphate-buffered saline (PBS group) or IL-32 (IL-32 group) were injected into both knee joints at day 28 and 32, then mice were killed at day 35. Severity of synovial inflammation and bone destruction was determined by histological scoring method, and synovial cytokine expressions such as IL-1β, TNF-α, IL-17, IL-18, IFN-γ, IL-21, and IL-23 were measured by real-time RT-PCR and western blot. Synovial NK cell expressions were determined by real-time RT-PCR, western blot and immunohistochemistry, and chemokines and chemokine receptors expressions that are associated with NK cell migration were determined by real-time RT-PCR. Scores of synovial inflammation and bone destruction, synovial expressions of IL-1β, TNF-α, IL-18, and IFN-γ were significantly increased in IL-32 group compared with PBS group. Synovial expressions of NK cell, and chemokines (CCL2 and CXCL9) and chemokine receptors (CCR2 and CCR5) that are associated with NK cell migration were significantly increased in IL-32 group compared with PBS group. IL-32 aggravated joint inflammation and bone destruction and increased synovial expressions of inflammatory cytokine and NK cells in CIA. These results suggest that IL-32 play a role in joint inflammation and bone destruction, and IL-32 might be a new target for treatment of rheumatoid arthritis.





Similar content being viewed by others
Abbreviations
- TNF:
-
Tumor necrosis factor
- IL:
-
Interleukin
- IFN:
-
Interferon
- NK cell:
-
Natural killer cell
- CIA:
-
Collagen-induced arthritis
- CII:
-
Type II collagen
- PBS:
-
Phosphate-buffered saline
- GM-CSF:
-
Granulocyte-macrophage colony-stimulating factors
References
Firestein GS (2003) Evolving concept of rheumatoid arthritis. Nature 423:356–361
Senolt L, Vencovsky J, Pavelka K, Ospelt C, Gay S (2009) Prospective new biological therapies for rheumatoid arthritis. Autoimmun Rev 9(2):102–107
Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA (2005) Interleukin-32: a cytokine and inducer of TNFalpha. Immunity 22:131–142
Joosten LA, Netea MG, Kim SH, Yoon DY, Oppers-Walgreen B, Radstake TR, Barrera P, van de Loo FA, Dinarello CA, van den Berg WB (2006) IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc Natl Acad Sci USA 103:3298–3303
Conti P, Youinou P, Theoharides TC (2007) Modulation of autoimmunity by the latest interleukins (with special emphasis on IL-32). Autoimmun Rev 6(3):131–137
Dinarello CA, Kim SH (2006) IL-32, a novel cytokine with a possible role in disease. Ann Rheum Dis 65(Suppl 3):iii61–iii64
Flodstrom-Tullberg M, Bryceson YT, Shi FD, Hoglund P, Liunggren HG (2009) Natural killer cells in human autoimmunity. Curr Opin Immunol 21(6):634–640
Yabuhara A, Yang FC, Nakazawa T, Iwasaki Y, Mori T, Koike K, Kawai H, Komiyama A (1996) A killing defect of natural killer cells as an underlying immunologic abnormality in childhood systemic lupus erythematosus. J Rheumatol 23:171–177
Cameron AL, Kirby B, Griffiths CE (2003) Circulating natural killer cells in psoriasis. Br J Dermatol 149:160–164
O’Gorman M, Smith R, Garrison A, Shamiyeh E, Pachman L (2002) Lymphocyte subsets in peripheral blood from newly diagnosed, untreated patients with juvenile dermatomyositis (JDM) are associated with disease activity scores (DAS). Arthr Rheum 46(suppl 9):S490
Grom AA, Villanueva J, Lee S, Goldmuntz EA, Passo MH, Filipovich A (2003) Natural killer cell dysfunction in patients with systemic-onset juvenile rheumatoid arthritis and macrophage activation syndrome. J Pediatr 142:292–296
Shibatomi K, Ida H, Yamasaki S, Nakashima T, Origuchi T, Kawakami A, Migita K, Kawabe Y, Tsujihata M, Anderson P, Eguchi K (2001) A novel role for interleukin-18 in human natural killer cell death: high serum levels and low natural killer cell numbers in patients with systemic autoimmune diseases. Arthr Rheum 44(4):884–892
Lo CK, Lam QL, Sun L, Wang S, Ko KH, Xu H, Wu CY, Zheng BJ, Lu L (2008) Natural killer cell degeneration exacerbates experimental arthritis in mice via enhanced interleukin-17 production. Arthr Rheum 58(9):2700–2711
Shoda H, Fujio K, Yamamoto K (2007) Rheumatoid arthritis and interleukin-32. Cell Mol Life Sci 64:2671–2679
Kim YG, Lee CK, Oh JS, Kim SH, Kim KA, Yoo B (2010) Effect of interleukin-32gamma on differentiation of osteoclasts from CD14+ monocytes. Arthr Rheum 62(2):515–523
Mabilleau G, Sabokbar A (2009) Interleukin-32 promotes osteoclast differentiation but not osteoclast activation. PLoS ONE 4(1):e4173
Harris ED (1990) Rheumatoid arthritis: pathophysiology and implications for treatment. N Engl J Med 322:1277–1289
Davis LS, Schulze-Koops H, Lipsky PE (1999) Human CD4+ T cell differentiation and effector function: implications for autoimmunity. Immunol Res 19(1):25–34
Kotzin BL, Kappler J (1998) Targeting the T cell receptor in rheumatoid arthritis. Arthr Rheum 41:1906–1910
Abbas AK, Murphy KM, Sher A (1996) Functional diversity of helper T lymphocytes. Nature 383:787–793
Mosmann TR, Sad S (1996) The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today 17:138–146
Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D (2006) IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest 116:1310–1316
Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, Martin TJ, Suda T (1999) IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest 103:1345–1352
Kohno K, Kurimoto M (1998) Interleukin 18, a cytokine which resembles IL-1 structurally and IL-12 functionally but exerts its effect independently of both. Clin Immunol Immunopathol 86(1):11–15
Udagewa N, Horwood NJ, Elliott J, Mackay A, Owens J, Okamura H, Kurimoto M, Chambers TJ, Martin TJ, Gillespie MT (1997) Interleukin-18 (interferon-gamma-inducing factor) is produced by osteoblasts and acts via granulocyte/macrophage colony-stimulating factor and not via interferon-gamma to inhibit osteoclast formation. J Exp Med 185(6):1005–1012
Olee T, Hashimoto S, Quach J, Lotz M (1999) IL-18 is produced by articular chondrocytes and induces proinflammatory and catabolic responses. J Immunol 162(2):1096–1100
Okamura H, Kashiwamura S, Tsutsui H, Yoshimoto T, Nakanishi K (1998) Regulation of interferon-gamma production by IL-12 and IL-18. Curr Opin Immunol 10(3):258–264
Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S (1998) Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity 8(3):383–390
Gracie JA, Forsey RJ, Chan WL, Gilmour A, Leung BP, Greer MR, Kennedy K, Carter R, Wei XQ, Xu D, Field M, Foulis A, Liew FY, Mclnners IB (1999) A proinflammatory role for IL-18 in rheumatoid arthritis. J Clin Invest 104(10):1393–1401
Yokoyama WM (1998) Natural killer cell receptors. Curr Opin Immunol 10:298–305
Cooper MA, Fehniger TA, Caligiuri MA (2001) The biology of human natural killer-cell subsets. Trends Immunol 22:633–640
Robertson MJ, Ritz J (1990) Biology and clinical relevance of human natural killer cells. Blood 76:2421–2438
Fearon DT, Locksley RM (1996) The instructive role of innate immunity in the acquired immune response. Science 272:50–53
Kos FJ (1998) Regulation of adaptive immunity by natural killer cells. Immunol Res 17:303–312
Su HC, Nguyen KB, Salazar-Mather TP, Ruzek MC, Dalod MY, Biron CA (2001) NK cell functions restrain T cell responses during viral infections. Eur J Immunol 31:3048–3055
Zitvogel L (2002) Dendritic and natural killer cells cooperate in the control/switch of innate immunity. J Exp Med 195:F9–F14
Zhang B, Yamamura T, Kondo T, Fujiwara M, Tabira T (1997) Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J Exp Med 186:1677–1687
Nilsson N, Bremell T, Tarkowski A, Carlsten H (1999) Protective role of NK1.1+ cells in experimental Staphylococcus aureus arthritis. Clin Exp Immunol 117:63–69
Charo IF, Ransohoff RM (2006) The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 354(6):610–621
Szekanecz Z, Kim J, Koch AE (2003) Chemokines and chemokine receptors in rheumatoid arthritis. Semin Immunol 15(1):15–21
Conflict of interest
The authors declare that they have no conflicts of interest concerning this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, YE., Kim, GT., Lee, SG. et al. IL-32 aggravates synovial inflammation and bone destruction and increases synovial natural killer cells in experimental arthritis models. Rheumatol Int 33, 671–679 (2013). https://doi.org/10.1007/s00296-012-2385-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-012-2385-5