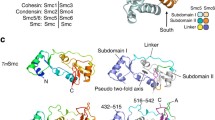
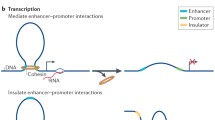
Abstract
The higher-order organization of chromosomes ensures their stability and functionality. However, the molecular mechanism by which higher order structure is established is poorly understood. Dissecting the activity of the relevant proteins provides information essential for achieving a comprehensive understanding of chromosome structure. Proteins of the structural maintenance of chromosome (SMC) family of ATPases are the core of evolutionary conserved complexes. SMC complexes are involved in regulating genome dynamics and in maintaining genome stability. The structure of all SMC proteins resembles an elongated rod that contains a central coiled-coil domain, a common protein structural motif in which two α-helices twist together. In recent years, the imperative role of the coiled-coil domain to SMC protein activity and regulation has become evident. Here, we discuss recent advances in the function of the SMC coiled coils. We describe the structure of the coiled-coil domain of SMC proteins, modifications and interactions that are mediated by it. Furthermore, we assess the role of the coiled-coil domain in conformational switches of SMC proteins, and in determining the architecture of the SMC dimer. Finally, we review the interplay between mutations in the coiled-coil domain and human disorders. We suggest that distinctive properties of coiled coils of different SMC proteins contribute to their distinct functions. The discussion clarifies the mechanisms underlying the activity of SMC proteins, and advocates future studies to elucidate the function of the SMC coiled coil domain.


Similar content being viewed by others
References
Anderson DE, Losada A, Erickson HP, Hirano T (2002) Condensin and cohesin display different arm conformations with characteristic hinge angles. J Cell Biol 156:419–424
Andrews EA, Palecek J, Sergeant J, Taylor E, Lehmann AR, Watts FZ (2005) Nse2, a component of the Smc5-6 complex, is a SUMO ligase required for the response to DNA damage. Mol Cell Biol 25:185–196
Barysz H, Kim JH, Chen ZA, Hudson DF, Rappsilber J, Gerloff DL, Earnshaw WC (2015) Three-dimensional topology of the SMC2/SMC4 subcomplex from chicken condensin I revealed by cross-linking and molecular modelling. Open Biol 5:150005
Beasley M, Xu H, Warren W, McKay M (2002) Conserved disruptions in the predicted coiled-coil domains of eukaryotic SMC complexes: implications for structure and function. Genome Res 12:1201–1209
Burkhard P, Steinmetz MO, Schulthess T, Landwehr R, Aebi U, Kammerer RA (1998) Crystallization and preliminary X-ray diffraction analysis of the 190-A-long coiled-coil dimerization domain of the actin-bundling protein cortexillin I from dictyostelium discoideum. J Struct Biol 122:293–296
Burmann F, Basfeld A, Vazquez Nunez R, Diebold-Durand ML, Wilhelm L, Gruber S (2017) Tuned SMC arms drive chromosomal loading of prokaryotic condensin. Mol Cell 65(861–872):e869
Chan KL, Roig MB, Hu B, Beckouet F, Metson J, Nasmyth K (2012) Cohesin’s DNA exit gate is distinct from its entrance gate and is regulated by acetylation. Cell 150:961–974
Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M (2009) Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325:834–840
Deardorff MA, Noon SE, Krantz ID (1993) Cornelia de Lange syndrome. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Ledbetter N, Mefford HC, Smith RJH et al (eds) GeneReviews®. University of Washington, Seattle, WA, USA
Dephoure N, Zhou C, Villen J, Beausoleil SA, Bakalarski CE, Elledge SJ, Gygi SP (2008) A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci USA 105:10762–10767
Diebold-Durand ML, Lee H, Ruiz Avila LB, Noh H, Shin HC, Im H, Bock FP, Burmann F, Durand A, Basfeld A et al (2017) Structure of full-length SMC and rearrangements required for chromosome organization. Mol Cell 67(334–347):e335
Ding DQ, Haraguchi T, Hiraoka Y (2016) A cohesin-based structural platform supporting homologous chromosome pairing in meiosis. Curr Genet 62:499–502
Duan X, Yang Y, Chen YH, Arenz J, Rangi GK, Zhao X, Ye H (2009) Architecture of the Smc5/6 complex of Saccharomyces cerevisiae reveals a unique interaction between the Nse5-6 subcomplex and the hinge regions of Smc5 and Smc6. J Biol Chem 284:8507–8515
Duan X, Holmes WB, Ye H (2011) Interaction mapping between Saccharomyces cerevisiae Smc5 and SUMO E3 ligase Mms21. Biochemistry 50:10182–10188
Eeftens JM, Katan AJ, Kschonsak M, Hassler M, de Wilde L, Dief EM, Haering CH, Dekker C (2016) Condensin Smc2-Smc4 dimers are flexible and dynamic. Cell Rep 14:1813–1818
Fennell-Fezzie R, Gradia SD, Akey D, Berger JM (2005) The MukF subunit of Escherichia coli condensin: architecture and functional relationship to kleisins. EMBO J 24:1921–1930
Forbes SA, Beare D, Boutselakis H, Bamford S, Bindal N, Tate J, Cole CG, Ward S, Dawson E, Ponting L et al (2017) COSMIC: somatic cancer genetics at high-resolution. Nucleic Acids Res 45:D777–D783
Gil-Rodriguez MC, Deardorff MA, Ansari M, Tan CA, Parenti I, Baquero-Montoya C, Ousager LB, Puisac B, Hernandez-Marcos M, Teresa-Rodrigo ME et al (2015) De novo heterozygous mutations in SMC3 cause a range of Cornelia de Lange syndrome-overlapping phenotypes. Hum Mutat 36:454–462
Gligoris TG, Scheinost JC, Burmann F, Petela N, Chan KL, Uluocak P, Beckouet F, Gruber S, Nasmyth K, Lowe J (2014) Closing the cohesin ring: structure and function of its Smc3-kleisin interface. Science 346:963–967
Griese JJ, Witte G, Hopfner KP (2010) Structure and DNA binding activity of the mouse condensin hinge domain highlight common and diverse features of SMC proteins. Nucleic Acids Res 38:3454–3465
Haering CH, Lowe J, Hochwagen A, Nasmyth K (2002) Molecular architecture of SMC proteins and the yeast cohesin complex. Mol Cell 9:773–788
Hill VK, Kim JS, Waldman T (2016) Cohesin mutations in human cancer. Biochem Biophys Acta 1866:1–11
Hirano M, Hirano T (2002) Hinge-mediated dimerization of SMC protein is essential for its dynamic interaction with DNA. EMBO J 21:5733–5744
Hirano M, Hirano T (2006) Opening closed arms: long-distance activation of SMC ATPase by hinge-DNA interactions. Mol Cell 21:175–186
Huis PJ, Herzog F, Ladurner R, Davidson IF, Piric S, Kreidl E, Bhaskara V, Aebersold R, Peters JM (2014) Characterization of a DNA exit gate in the human cohesin ring. Science 346:968–972
Huisman S, Mulder PA, Redeker E, Bader I, Bisgaard AM, Brooks A, Cereda A, Cinca C, Clark D, Cormier-Daire V et al (2017) Phenotypes and genotypes in individuals with SMC1A variants. Am J Med Genetics A 173:2108–2125
Iwasaki O, Noma KI (2016) Condensin-mediated chromosome organization in fission yeast. Curr Genet 62:739–743
Kammerer RA (1997) Alpha-helical coiled-coil oligomerization domains in extracellular proteins. Matrix Biol 15:555–565
Kikuchi S, Borek DM, Otwinowski Z, Tomchick DR, Yu H (2016) Crystal structure of the cohesin loader Scc2 and insight into cohesinopathy. Proc Natl Acad Sci USA 113:12444–12449
Kohn WD, Mant CT, Hodges RS (1997) Alpha-helical protein assembly motifs. J Biol Chem 272:2583–2586
Kulemzina I, Ang K, Zhao X, Teh JT, Verma V, Suranthran S, Chavda AP, Huber RG, Eisenhaber B, Eisenhaber F et al (2016) A reversible association between Smc coiled coils is regulated by lysine acetylation and is required for cohesin association with the DNA. Mol Cell 63:1044–1054
Lehmann AR, Walicka M, Griffiths DJ, Murray JM, Watts FZ, McCready S, Carr AM (1995) The rad18 gene of Schizosaccharomyces pombe defines a new subgroup of the SMC superfamily involved in DNA repair. Mol Cell Biol 15:7067–7080
Li Y, Schoeffler AJ, Berger JM, Oakley MG (2010) The crystal structure of the hinge domain of the Escherichia coli structural maintenance of chromosomes protein MukB. J Mol Biol 395:11–19
Losada A (2014) Cohesin in cancer: chromosome segregation and beyond. Nat Rev Cancer 14:389–393
Lupas A (1996) Coiled coils: new structures and new functions. Trends Biochem Sci 21:375–382
Mannini L, Cucco F, Quarantotti V, Krantz ID, Musio A (2013) Mutation spectrum and genotype–phenotype correlation in Cornelia de Lange syndrome. Hum Mutat 34:1589–1596
Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER 3rd, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y et al (2007) ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 316:1160–1166
Milutinovich M, Unal E, Ward C, Skibbens RV, Koshland D (2007) A multi-step pathway for the establishment of sister chromatid cohesion. PLoS Genet 3:e12
Murayama Y, Uhlmann F (2014) Biochemical reconstitution of topological DNA binding by the cohesin ring. Nature 505:367–371
Nasmyth K, Haering CH (2005) The structure and function of SMC and kleisin complexes. Annu Rev Biochem 74:595–648
Nolivos S, Sherratt D (2014) The bacterial chromosome: architecture and action of bacterial SMC and SMC-like complexes. FEMS Microbiol Rev 38:380–392
Olsen JV, Vermeulen M, Santamaria A, Kumar C, Miller ML, Jensen LJ, Gnad F, Cox J, Jensen TS, Nigg EA, Brunak S, Mann M (2010) Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci Signal 3(104):ra3. doi:10.1126/scisignal.2000475
Onn I, Aono N, Hirano M, Hirano T (2007) Reconstitution and subunit geometry of human condensin complexes. EMBO J 26:1024–1034
Onn I, Heidinger-Pauli JM, Guacci V, Unal E, Koshland DE (2008) Sister chromatid cohesion: a simple concept with a complex reality. Annu Rev Cell Dev Biol 24:105–129
Orgil O, Matityahu A, Eng T, Guacci V, Koshland D, Onn I (2015) A conserved domain in the scc3 subunit of cohesin mediates the interaction with both mcd1 and the cohesin loader complex. PLoS Genet 11:e1005036
Orgil O, Mor H, Matityahu A, Onn I (2016) Identification of a region in the coiled-coil domain of Smc3 that is essential for cohesin activity. Nucleic Acids Res 44:6309–6317
Ouyang Z, Yu H (2017) Releasing the cohesin ring: a rigid scaffold model for opening the DNA exit gate by Pds5 and Wapl. BioEssays 39(4). doi:10.1002/bies.201600207
Peterson TA, Gauran IIM, Park J, Park D, Kann MG (2017) Oncodomains: a protein domain-centric framework for analyzing rare variants in tumor samples. PLoS Comput Biol 13:e1005428
Potts PR, Yu H (2005) Human MMS21/NSE2 is a SUMO ligase required for DNA repair. Mol Cell Biol 25:7021–7032
Revenkova E, Focarelli ML, Susani L, Paulis M, Bassi MT, Mannini L, Frattini A, Delia D, Krantz I, Vezzoni P et al (2009) Cornelia de Lange syndrome mutations in SMC1A or SMC3 affect binding to DNA. Hum Mol Genet 18:418–427
Rolef Ben-Shahar T, Heeger S, Lehane C, East P, Flynn H, Skehel M, Uhlmann F (2008) Eco1-dependent cohesin acetylation during establishment of sister chromatid cohesion. Science 321:563–566
Sergeant J, Taylor E, Palecek J, Fousteri M, Andrews EA, Sweeney S, Shinagawa H, Watts FZ, Lehmann AR (2005) Composition and architecture of the Schizosaccharomyces pombe Rad18 (Smc5-6) complex. Mol Cell Biol 25:172–184
Shwartz M, Matityahu A, Onn I (2016) Identification of functional domains in the cohesin loader subunit Scc4 by a random insertion/dominant negative screen. G3 6:2655–2663
Uhlmann F (2016) SMC complexes: from DNA to chromosomes. Nat Rev Mol Cell Biol 17:399–412
Unal E, Heidinger-Pauli JM, Kim W, Guacci V, Onn I, Gygi SP, Koshland DE (2008) A molecular determinant for the establishment of sister chromatid cohesion. Science 321:566–569
Waldman VM, Stanage TH, Mims A, Norden IS, Oakley MG (2015) Structural mapping of the coiled-coil domain of a bacterial condensin and comparative analyses across all domains of life suggest conserved features of SMC proteins. Proteins 83:1027–1045
Weitzel CS, Waldman VM, Graham TA, Oakley MG (2011) A repeated coiled-coil interruption in the Escherichia coli condensin MukB. J Mol Biol 414:578–595
Woo JS, Lim JH, Shin HC, Suh MK, Ku B, Lee KH, Joo K, Robinson H, Lee J, Park SY et al (2009) Structural studies of a bacterial condensin complex reveal ATP-dependent disruption of intersubunit interactions. Cell 136:85–96
Yoshimura SH, Hizume K, Murakami A, Sutani T, Takeyasu K, Yanagida M (2002) Condensin architecture and interaction with DNA: regulatory non-SMC subunits bind to the head of SMC heterodimer. Current Biol 12:508–513
Zhao X, Blobel G (2005) A SUMO ligase is part of a nuclear multiprotein complex that affects DNA repair and chromosomal organization. Proc Natl Acad Sci USA 102:4777–4782
Acknowledgements
We would like to thank members of the Onn lab for discussing the subject. This work was supported by grants from Israel Cancer Association 20170111 and Israel Science Foundation Grant 1099/16 to I.O.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kupiec.
Rights and permissions
About this article
Cite this article
Matityahu, A., Onn, I. A new twist in the coil: functions of the coiled-coil domain of structural maintenance of chromosome (SMC) proteins. Curr Genet 64, 109–116 (2018). https://doi.org/10.1007/s00294-017-0735-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-017-0735-2